Key Points
Routine staging by PET-CT identifies all clinically relevant marrow involvement by DLBCL.
Cases with marrow involvement identified by PET-CT have PFS and overall survival similar to stage IV cases without marrow involvement.
Abstract
We investigated whether positron emission tomography combined with computed tomography (PET-CT) identifies clinically important bone marrow involvement by diffuse large B-cell lymphoma (DLBCL) with sufficient accuracy to replace routine staging bone marrow biopsy. All patients from a single centre diagnosed as DLBCL since 2005 had data extracted from staging PET-CT, marrow biopsy, and treatment records. Of 130 patients, 35 (27%) were judged to have marrow involvement; 33 were identified by PET-CT compared with 14 by marrow histology. PET identified all clinically important marrow lymphoma, while biopsy did not upstage any patient. Sensitivity and specificity were 94% and 100% for PET-CT and 40% and 100% for marrow biopsy. As a secondary aim, we compared the prognosis of marrow involvement, as detected by PET-CT or biopsy. Cases with marrow deposits identified by PET-CT but not biopsy had progression-free survival (PFS) and overall survival similar to stage IV disease without involved marrow. Positive biopsy conferred significantly inferior PFS (P = .003); these cases frequently had other markers of poor-risk disease. These data confirm that in experienced hands PET-CT has a high level of accuracy for identifying marrow disease in DLBCL, and provide new insight into the nature and clinical significance of marrow involvement.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL). Disease involves the bone marrow in up to 27% of cases, as assessed by iliac crest bone marrow biopsy (BMB) at the time of diagnosis.1-3
Bone marrow involvement, categorized as an extranodal site, adversely affects stage and, consequently, the International Prognostic Index (IPI). Traditionally, bone marrow infiltration by lymphoma has been assessed by unilateral or bilateral iliac crest biopsy, and this currently remains the standard. Positron emission tomography (PET), increasingly combined with computed tomography (PET-CT), is now a routine part of staging DLBCL, owing to its ability to accurately evaluate both nodal and extranodal disease sites (including skeletal involvement), assign stage, and quantify prognosis.4-7 Recently, a large retrospective study of marrow disease in DLBCL cases treated with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) reported that histologic evidence of DLBCL in staging marrow biopsy adversely affected overall survival (OS) and event-free survival, independent of the IPI.8
We first examined PET as a method to assess bone marrow involvement in Hodgkin lymphoma (HL) and NHL in 19989 and reported that bone marrow involvement in both diseases may have a metastatic pattern, apparent as focal areas of increased 18F-fluorodeoxyglucose (FDG) uptake, or a more diffuse pattern of involvement with uniform FDG uptake throughout the marrow space. Although it is accepted that iliac crest biopsy misses a proportion of focal disease detected using PET, there is continuing debate as to whether PET is a sufficiently sensitive method of detecting marrow involvement by DLBCL to replace histologic marrow assessment.10 Part of the uncertainty relates to variable FDG avidity of other NHLs included in previous studies, as well as difficulty in the accurate anatomical localization of small areas of FDG uptake using PET as opposed to PET-CT.11-13
The debate has been given fresh focus by a recent study which concluded that in Hodgkin disease staged by PET, marrow biopsy added no therapeutically useful information.14 However, DLBCL is not directly analogous; marrow biopsy may reveal histologically discordant infiltration by low-grade lymphoma, and there has been prevalent clinical opinion that marrow disease per se is a risk factor for spread to the central nervous system (CNS), though the evidence for this association has recently been questioned.15-18
With our long experience of reporting bone marrow disease in DLBCL using PET and, for the past seven years, PET-CT, we wished to examine whether our diagnosis of marrow involvement by DLBCL, based on routine staging PET-CT, was sufficiently accurate to render staging BMB unnecessary.
A secondary purpose was to document the prognosis of cases with marrow involvement identified by half body PET-CT, and the prognosis of cases identified histologically by iliac crest biopsy, compared with stage IV cases without marrow involvement.
Methods
Study population and data extraction
PET-CT replaced PET in 2005 at Guy’s and St Thomas’ Hospital. All patients diagnosed with DLBCL at our institution are staged by PET-CT, and all DLBCL cases between January 2005 and January 2012 were included. The majority were treated with R-CHOP; 7 cases considered exceptionally high risk based on tumor genetics and/or aggressive HIV-related lymphoma were given more intensive chemotherapy (R-DHAP [rituximab, dexamethasone, cytarabine, cisplatin], or a high-dose methotrexate/cyclophosphamide regimen) as primary treatment.
Cases were retrospectively identified from our clinical database. Patients were excluded if they had received chemotherapy or glucocorticoids prior to PET-CT imaging. Clinical stage was based on routine clinical and laboratory assessments, PET-CT imaging, and unilateral iliac crest (BMB). All cases had imaging, histology, and clinical data reviewed at a weekly multidisciplinary meeting to assign stage, prognostic score, and plan treatment. For the purpose of this study, the presence or absence of bone marrow disease in each patient was determined from the routine histopathology and PET-CT reports. Treatment decisions were extracted from the multidisciplinary meeting records. Clinical case records were reviewed to assess disease status up to August 2012. Progression-free survival (PFS) and OS were calculated from diagnosis to disease progression or death from any cause.
Patient data were extracted from case records and reviewed only by members of the responsible clinical team, in compliance with the UK Data Protection Act; consequently, specific research ethics approval was not required.
Identification of marrow disease by PET
PET-CT imaging was performed using DST or VCT PET-CT scanners (General Electric), 90 minutes after administration of 370 MBq FDG. Patients were scanned from proximal femur to skull base, unless extended to cover an initial site of presentation outside this area. Scans were reported at diagnosis using a standard display scaled to a maximum standardized uptake value of 10, and read by 2 independent reviewers, always including a senior PET physician/radiologist with >10 years of PET reporting experience. Discrepancies were resolved by a third reader reviewing the scan. During the period of study, ∼1% of all scans reported by the department required a third read. The interobserver and intraobserver variation among readers in our department for staging lymphoma with PET-CT has been published, reporting high agreement for both nodal and extranodal disease, including bone marrow involvement.19
Marrow involvement by lymphoma was classified as focal only, diffuse only, or both focal and diffuse, as previously described by ourselves and recently by El-Galaly et al.9,14 Focal was defined as 1 or more circumscribed area(s) of high FDG uptake within the skeleton; diffuse was defined as uniform increased FDG uptake throughout the bone marrow space. Cases where bone marrow involvement appeared to be by contiguous spread from adjacent soft tissue disease were excluded from analysis.
All staging scans reported as showing bone or bone marrow involvement were re-examined by 1 of the authors (S.F.B.). In addition, scans of patients with reported histologic marrow disease but a marrow-negative PET report were reviewed. The reviewer was blind to the original scan and histology reports, and outcome data. To determine that FDG-avid lesions at diagnosis truly represented lymphoma deposits, cases with increased marrow FDG uptake on staging PET had review of the interim PET-CT scan routinely performed after 2 to 3 cycles of chemotherapy and, where available, the end of treatment scan. The three criteria for FDG uptake in bone marrow to be classified as due to DLBCL were for FDG uptake to be greater than the intensity of uptake in normal liver, with no anatomical changes to suggest alternative ‘benign’ bone pathology, and to have resolved in parallel with nodal disease during treatment, as has been previously reported.14
Identification of marrow disease by iliac crest biopsy
In our institution, unilateral iliac crest biopsy is standard. Marrow biopsy specimens were obtained within 1 month of staging PET-CT and reported by a senior hematopathologist. The presence of lymphoma was based on standard immunohistochemistry, including antibodies to identify CD3, CD79a, and CD20-positive cells.
Data analysis
Patients were considered to have bone marrow involvement by lymphoma if they had either histologic DLBCL in the marrow biopsy or PET-positive marrow involvement irrespective of iliac crest biopsy histology.
PFS and OS were calculated for patients identified as stage IV as follows: cases with marrow involvement identified by PET-CT (PET+) were compared with marrow PET-negative (PET–) stage IV cases; marrow biopsy-positive (BMB+) cases were compared with biopsy-negative (BMB–) cases. In addition, the subset of cases who had disease detected by PET, but not marrow histology (PET+/BM–), were compared with PET–/BM– stage IV cases.
Survival according to PET and BMB status were compared using Kaplan-Meier plots. Cox proportional hazards models were fitted to estimate hazard ratio (HR). Some confounding due to age, IPI, HIV status, and extensive extranodal disease (≥4 organ involvement) was expected in estimating the association between BMB status and survival. To investigate this, attempts were made to adjust HRs for these variables, but due to the low number of events per variable it was not possible to adjust for all simultaneously.20,21 Instead, HRs were adjusted for each variable individually. All analyses were done using Stata 12 (2011; StataCorp).
Results
One hundred thirty adult patients with DLBCL were identified for inclusion (median, age 59 years; range, 22-87 years). Six patients had previously documented follicular or marginal zone lymphoma (MZL) prior to presentation with high-grade transformation to DLBCL. Sixteen were HIV-positive (Table 1).
Cohort patient characteristics
| Characteristics . | No. (%) . |
|---|---|
| Study subjects, n | 130 |
| Gender M:F (%) | 77:53 (59:41) |
| Age, median (range), y | 59 (22-87) |
| HIV positive | 16 |
| Stage | |
| I | 30 (23) |
| II | 29 (22) |
| III | 26 (20) |
| IV | 45 (35) |
| IPI low (0-1) | 43 (33) |
| IPI low-intermediate (2) | 25 (19) |
| IPI high-intermediate (3) | 29 (22) |
| IPI high (4-5) | 17 (13) |
| IPI not available (missing data) | 16 (12) |
| Characteristics . | No. (%) . |
|---|---|
| Study subjects, n | 130 |
| Gender M:F (%) | 77:53 (59:41) |
| Age, median (range), y | 59 (22-87) |
| HIV positive | 16 |
| Stage | |
| I | 30 (23) |
| II | 29 (22) |
| III | 26 (20) |
| IV | 45 (35) |
| IPI low (0-1) | 43 (33) |
| IPI low-intermediate (2) | 25 (19) |
| IPI high-intermediate (3) | 29 (22) |
| IPI high (4-5) | 17 (13) |
| IPI not available (missing data) | 16 (12) |
Identification of marrow disease by PET and iliac crest biopsy
Based on integration of all imaging and histologic data, 35 (27%) patients were considered to have evidence of DLBCL in marrow at diagnosis: marrow disease was detected by PET-CT in 33 (94%) and histologically in 14 (40%) (Figure 1).
Case distribution of bone marrow status assessed by PET-CT and BM biopsy. BM, bone marrow; BMB, bone marrow biopsy.
Case distribution of bone marrow status assessed by PET-CT and BM biopsy. BM, bone marrow; BMB, bone marrow biopsy.
Marrow disease identified by PET had an exclusively focal pattern of FDG uptake in 28 cases; iliac crest biopsy failed to identify marrow involvement in 21 of these subjects. Uniformly diffuse increased FDG uptake throughout the skeletal marrow space was recorded in 5 patients: in 3 of these, there were additional focal areas of FDG uptake distant from the iliac crests, and all 5 had iliac crest histologic confirmation of marrow infiltration by DLBCL cells. No cases had cortical bone involvement without increased FDG uptake in the marrow space. Representative scans from patients with marrow involvement are shown (Figure 2).
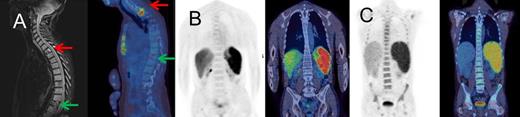
Example PET-CT images. (A) Focal FDG uptake in T2 on fused PET-CT sagittal image representing a DLBCL deposit (red arrow); no uptake at a compression fracture of T11 (green arrow) with confirmatory magnetic resonance image; iliac crest biopsy was negative. (B) Case with 10% diffuse DLBCL involvement on marrow histology, without increased FDG uptake in bone marrow. (C) Diffuse uptake throughout skeletal marrow, which was confirmed as DLBCL on marrow histology. The patient was HIV-positive with MYC and BCL2 abnormalities. PET and fused PET-CT coronal slices are shown.
Example PET-CT images. (A) Focal FDG uptake in T2 on fused PET-CT sagittal image representing a DLBCL deposit (red arrow); no uptake at a compression fracture of T11 (green arrow) with confirmatory magnetic resonance image; iliac crest biopsy was negative. (B) Case with 10% diffuse DLBCL involvement on marrow histology, without increased FDG uptake in bone marrow. (C) Diffuse uptake throughout skeletal marrow, which was confirmed as DLBCL on marrow histology. The patient was HIV-positive with MYC and BCL2 abnormalities. PET and fused PET-CT coronal slices are shown.
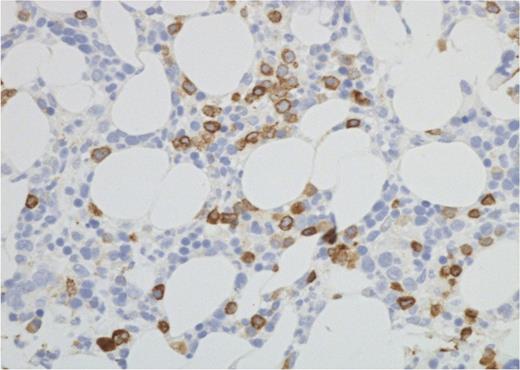
Marrow disease identified histologically showed DLBCL in all cases. No cases had discordant (low-grade) or mixed histology on staging biopsy, though 2 patients had follicular lymphoma (FL) identified on repeat biopsies later in their disease course. Marrow involvement by DLBCL had been correctly identified by PET in all but 2 cases; these had no marrow FDG uptake, but on histology had a diffuse infiltrate of ∼10% large B cells (Figure 3). It is of note that 1 of these cases was classified as equivocal on the routine PET-CT reporting but was subsequently classified as negative on review. Both cases had extranodal disease defining them as stage IV due to disease elsewhere.
Iliac crest bone marrow histology. Photomicrograph (CD79a immunohistochemistry) from 1 of the 2 PET−/BMB+ patients. The interstitial infiltrate by DLBCL was patchy, as in this field, but overall CD79a-positive cells amounted to ∼10% of all nucleated marrow cells (Nikon Eclipse 80i microscope and camera; ×40 objective).
Iliac crest bone marrow histology. Photomicrograph (CD79a immunohistochemistry) from 1 of the 2 PET−/BMB+ patients. The interstitial infiltrate by DLBCL was patchy, as in this field, but overall CD79a-positive cells amounted to ∼10% of all nucleated marrow cells (Nikon Eclipse 80i microscope and camera; ×40 objective).
Sensitivity and specificity for identifying marrow involvement were 94% and 100% for PET and 40% and 100% for BMB (overall accuracy: PET, 98.5%; BMB, 84%).
Bone marrow disease and staging
Forty-five of the 130-case cohort were classified as stage IV. Nine clinical stage I,II patients (who had no extranodal disease in soft tissue or detected by marrow biopsy) were classified as stage IV based solely on identification of marrow involvement by PET. Hence, PET identification of marrow disease increased the number of stage IV patients in the cohort by 25%. No case was classified as stage IV based on BMB alone.
Bone marrow disease and CNS prophylaxis
It is not our policy to routinely give CNS prophylaxis to patients with marrow involvement, however identified. Of the 35 patients identified as having marrow disease, 21 (8 BMB+, 13 BMB−) were given prophylactic CNS-directed therapy (these decisions being based on high-risk indicators or site of disease other than bone marrow involvement) and 3 developed CNS disease. The remaining 14 cases (6 BMB+, 8 BMB−) had no CNS prophylaxis; none developed CNS disease. Our policy and these data are in line with recent published evidence that a positive marrow biopsy is not an independent risk factor for spread to CNS in the rituximab era.15
Survival of cases with stage IV disease
Forty-four cases with stage IV disease at diagnosis were subject to survival analysis: 35 (79%) had marrow involvement, 9 had extranodal disease in soft tissue only. An additional stage IV case, without marrow involvement, was lost to follow-up.
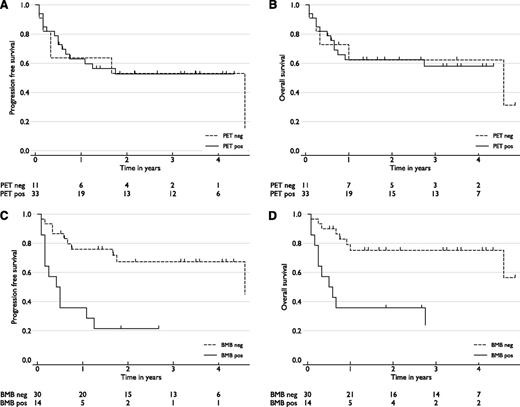
Patients with marrow involvement identified by PET (PET+) had similar PFS and OS to stage IV cases without marrow involvement (PET–). This was equally true whether the comparison included all PET+ cases (n = 33) (HRs: PFS, 0.8 [95% confidence interval (CI), 0.3-2.0] not significant [ns]; OS, 0.9 (0.3-2.5) ns) (Figure 4A-B), or only the subset of cases who were PET+/BMB– (n = 21) (PFS, 0.36 [0.1-1.2] ns; OS, 0.4 [0.1-1.6] ns) (supplemental Figure 1, available on the Blood website). In contrast, the 14 cases with positive marrow biopsy histology (BMB+) had significantly inferior outcomes compared with biopsy-negative (BMB–) cases (HRs: PFS, 3.7 [1.6-8.8], P = .003; OS, 3.9 [1.5-10.0], P = .004) (Figure 4C-D).
(A-D) PFS and OS analysis of stage IV patients, by marrow status. PFS and OS of cases identified by (A-B) PET-CT (PET) or (C-D) marrow biopsy (BMB) histology. Marrow PET-positive cases had outcomes similar to marrow PET-negative stage IV cases. Cases with DLBCL cells identified in marrow histologically had inferior survivals (P < .01). The number of cases in each group, and at risk at each follow up time point, are shown.
(A-D) PFS and OS analysis of stage IV patients, by marrow status. PFS and OS of cases identified by (A-B) PET-CT (PET) or (C-D) marrow biopsy (BMB) histology. Marrow PET-positive cases had outcomes similar to marrow PET-negative stage IV cases. Cases with DLBCL cells identified in marrow histologically had inferior survivals (P < .01). The number of cases in each group, and at risk at each follow up time point, are shown.
These 14 BMB+ cases were notable for the relative frequency of high-risk disease characteristics, including HIV, multiple extranodal disease sites (≥4 separate organs involved), and high-risk gene rearrangements (IGH/MYC, or IGH/MYC with IGH/BCL2).22,23 Multivariable analysis was attempted to explore whether the poor outcome of BMB-positive cases could be explained by IPI (Table 2), or 1 or more of these poor-risk indicators (Table 3). Due to the small number of events (disease progression or death) per variable, it was not possible to adjust for these suspected confounders simultaneously.20,21 Adjusting estimates for each confounder individually was similarly constrained and showed the HRs to be surprisingly consistent with each other and with the unadjusted HR. With HIV-positive cases excluded, for example, there was an improvement in PFS and OS of BMB-positive cases which did not reach significance, but there was no effect on survival of other stage IV cases (supplemental Figure 2). Consequently, limited by the small number of BMB+ cases, we were unable to demonstrate whether the poor outcome associated with a positive biopsy was or was not explicable by other high-risk disease indicators, either in combination or individually.
IPI distribution of stage IV cases with survival data, by marrow status identified by PET or BMB
| . | BM involvement identified by PET-CT (%) . | BM involvement identified by biopsy (%) . | ||
|---|---|---|---|---|
| PET+ . | PET– . | BMB+ . | BMB– . | |
| n | 33 | 11 | 14 | 30 |
| IPI 1 | 1 (3) | 0 | 0 | 1 (3) |
| IPI 2 | 6 (18) | 1 (9) | 2 (14) | 5 (17) |
| IPI 3 | 15 (46) | 4 (36) | 7 (50) | 12 (40) |
| IPI 4 | 7 (21) | 6 (55) | 4 (29) | 9 (30) |
| IPI 5 | 4 (12) | 0 | 1 (7) | 3 (10) |
| . | BM involvement identified by PET-CT (%) . | BM involvement identified by biopsy (%) . | ||
|---|---|---|---|---|
| PET+ . | PET– . | BMB+ . | BMB– . | |
| n | 33 | 11 | 14 | 30 |
| IPI 1 | 1 (3) | 0 | 0 | 1 (3) |
| IPI 2 | 6 (18) | 1 (9) | 2 (14) | 5 (17) |
| IPI 3 | 15 (46) | 4 (36) | 7 (50) | 12 (40) |
| IPI 4 | 7 (21) | 6 (55) | 4 (29) | 9 (30) |
| IPI 5 | 4 (12) | 0 | 1 (7) | 3 (10) |
The proportion of stage IV cases with each risk factor by marrow status identified by PET or BMB
| Risk factor . | BM involvement identified by PET-CT (%) . | BM involvement identified by biopsy (%) . | ||
|---|---|---|---|---|
| PET+ . | PET– . | BMB+ . | BMB– . | |
| Age, >60 y | 13/33 (39) | 6/11 (55) | 5/14 (36) | 14/30 (47) |
| IPI 3-5 | 26/33 (79) | 10/11 (91) | 12/14 (86) | 24/30 (80) |
| HIV-positive | 7/33 (21) | 2/11 (18) | 4/14 (29) | 5/30 (17) |
| ≥4 organ sites* of extranodal disease | 8/33 (24) | 3/11 (27) | 6/14 (43) | 5/30 (17) |
| High-risk gene rearrangement† | 6/17‡ (35) | 2/7‡ (28) | 6/9‡ (67) | 2/15‡ (13) |
| Risk factor . | BM involvement identified by PET-CT (%) . | BM involvement identified by biopsy (%) . | ||
|---|---|---|---|---|
| PET+ . | PET– . | BMB+ . | BMB– . | |
| Age, >60 y | 13/33 (39) | 6/11 (55) | 5/14 (36) | 14/30 (47) |
| IPI 3-5 | 26/33 (79) | 10/11 (91) | 12/14 (86) | 24/30 (80) |
| HIV-positive | 7/33 (21) | 2/11 (18) | 4/14 (29) | 5/30 (17) |
| ≥4 organ sites* of extranodal disease | 8/33 (24) | 3/11 (27) | 6/14 (43) | 5/30 (17) |
| High-risk gene rearrangement† | 6/17‡ (35) | 2/7‡ (28) | 6/9‡ (67) | 2/15‡ (13) |
At least 3 separate extranodal disease sites, plus bone marrow involvement.
IGH/MYC and double hit (IGH/MYC+IGH/BCL2) rearrangements.
Lymphoma genetics screening only became routine during the second half of the study period.
Discussion
The results from this single-center cohort study show that PET-CT can have a high level of accuracy for detecting bone marrow involvement in patients with DLBCL. PET-CT correctly diagnosed all clinically significant marrow involvement identifiable by marrow histology; in addition, PET-CT identified focal deposits of DLBCL not detected by iliac crest biopsy.
Difficulty in the correct interpretation of diffuse FDG uptake in bone marrow, leading to false-positives and poor specificity previously reported in HL and NHL24,25 was not evident in this study focused on DLBCL, suggesting that experienced reporters using a standardized display and strict reporting criteria are able to differentiate between infiltrating lymphoma and low-level “reactive” marrow FDG uptake. PET-CT failed to detect a low-volume large-cell infiltrate in 2 cases, which were already classified as stage IV due to extranodal disease elsewhere.
The limitations of the study are that it is retrospective and there is no practical method for defining the true incidence of bone marrow disease. Not all lesions identified by PET can be biopsied, and magnetic resonance imaging assessment of all lesions is not practical or justifiable if it will not affect treatment. Unilateral iliac crest BMB, as practiced by many lymphoma treatment centers, misses a significant proportion of marrow disease compared with bilateral sampling.26 We are confident that the focal FDG uptake interpreted here as lymphoma does represent metastatic lymphomatous deposits, based on accurate co-registration of metabolic and anatomical imaging, reduction of abnormal increased FDG accumulation following treatment in parallel with nodal response, and our experience in the early days of PET when biopsy was used to sample suspected marrow disease. Based on these criteria, in this cohort of 130 sequential patients diagnosed over 7 years, routinely reported PET-CT diagnosis of bone marrow involvement by DLBCL had no false-positives and only 2 false-negatives.
Since our first investigation exploring the accuracy of PET for identifying marrow disease in lymphoma,9 there have been a number of studies examining PET for detecting marrow disease in both indolent and aggressive lymphomas. These have been drawn together in 2 meta-analyses.25,27 Both reported on aggressive NHL as an aggregated subgroup and found similar sensitivity (76%, 72%) for PET detection of marrow disease. However, both analyses were hampered by the variety of different histologic classification schemes used and by use of PET alone in older studies. There is a paucity of reports analyzing data from DLBCL cases as a separate disease. A recent dual-center study comparing PET-CT and bilateral BMB for marrow staging identified 120 cases of DLBCL, 25 with marrow disease, and reported PET sensitivity and specificity of 84% and 100%, respectively.28
Our study demonstrates that even in the most experienced hands PET-CT may miss low-level marrow involvement by DLBCL, estimated at 10% of nucleated marrow cells in the 2 cases reported here. Others have shown that the extent of marrow infiltration is the most important factor influencing prognosis1 ; in a study to address this issue, 28 DLBCL patients with ≤10% marrow infiltrates had event-free survival no different from those without any histologic marrow disease.3
A reason given for marrow biopsy in DLBCL is to detect histologically discordant disease. PET will inevitably fail to identify indolent lymphoma in marrow if FDG avidity is low. Although 6 cases in our series had a history of FL or MZL preceding the diagnosis of DLBCL, or had mixed histology on lymph node biopsy, none had indolent NHL identified in the staging marrow biopsy. Paone et al29 correctly drew attention to this issue in a series of 97 DLBCL cases: 14 had small B-cell or small cleaved cell lymphoma in staging marrow biopsy, 10 of which had normal marrow FDG uptake and distribution on PET. The case was argued in this study for the need to continue histologic marrow assessment so as not to miss involvement by low-grade lymphoma.
Two clinicopathological studies2,26 have examined the prognostic significance of low-grade lymphoma as an incidental finding in marrow biopsy of DLBCL cases. Both conclude that response to treatment and survival does not differ between patients with marrow involved by low-grade lymphoma and patients with no marrow disease. A clinical study30 examined the natural history of patients, who at first diagnosis of DLBCL had evidence, on lymph node biopsy, of coexisting or transformed MZL, FL, or small cell lymphoma. These patients were older and most had disseminated disease with >1 extranodal site at staging. Complete remissions were fewer than in de novo DLBCL cases, and indolent disease relapses more common, but there was no significant difference in OS at 5 years. A more recent database study from British Columbia and New Zealand8 corroborated this and added evidence that indolent lymphoma found in bone marrow of patients with DLBCL had outcomes that were not independent of the IPI or its components.
The other reported finding of Sehn et al8 was that concordant bone marrow involvement by DLBCL, identified histologically, had a marked adverse impact on OS and PFS. However, a drawback of this important study of marrow histology is that it did not seek to take account of cases which would have had focal marrow disease identifiable by PET but not identified by biopsy.
As a secondary study objective, we investigated whether these 2 different patterns of marrow involvement had similar outcomes. We independently compared the outcomes of cases with marrow disease identified by PET-CT, and the outcomes of cases identified histologically by iliac crest biopsy, against stage IV cases without marrow involvement.
Focal (metastatic) deposits in bone marrow identified by PET-CT conferred a prognosis equivalent to stage IV extranodal disease in soft tissue. Conversely, marrow disease, identified by both marrow biopsy and PET-CT, was associated with significantly worse OS and event free survival.
The most plausible explanation for this difference would seem to be the extent of disease dissemination, with PET having a high sensitivity for limited metastatic disease but biopsy likely to detect disease in only the most extensively infiltrated marrow. Our study cohort provides some observational support for this, in that 8 of the 10 biopsy-positive patients who died had extranodal disease identified in 4 or more separate organs and/or diffuse FDG uptake throughout the skeletal marrow. However, this hypothesis needs to be tested in a larger cohort.
Conclusions and practical implications
These data from a single center confirm that in experienced hands PET-CT has a high level of sensitivity and accuracy for identifying marrow disease in DLBCL, and have provided new insight into the nature and clinical significance of marrow involvement by DLBCL.
We suggest this has practical implications for the staging of DLBCL as follows: cases without evidence of marrow disease on PET-CT are unlikely to have clinically significant marrow involvement detected by routine biopsy. Cases with limited focal deposits distant from the iliac crests are similarly unlikely to gain from marrow biopsy. Although not a diagnostic issue in this series, cases with a diffuse increase in FDG uptake throughout skeletal marrow, particularly in the absence of other poor-risk features, should have an iliac crest biopsy to establish whether this represents DLBCL, rather than FDG uptake by reactive myelopoiesis or other pathology.
Whether a positive marrow biopsy identifies cases with poor prognosis, over and above those already classified as high risk by routine noninvasive staging procedures, needs further study. In the meantime, we suggest that the “torture”31 of the routine marrow biopsy may no longer be necessary for all patients with DLBCL staged in experienced PET-CT centers.
Presented in part at the 11th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 15-18, 2011.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Paul Fields, Bridget Wilkins, Jon van der Walt, Mufaddal Moonim, and Michael Neat, whose meticulous clinical and diagnostic work made the study possible.
R.C. is funded in part by the King’s College London Academic Health Sciences Centre.
Authorship
Contribution: A.B.K. and R.C. devised the study; A.B.K., S.F.B., A.A.H., and L.C. contributed to collection, review, and/or analysis of the data; T.M. performed statistical analysis of the data; and R.C., A.B.K., S.F.B., N.G.M., and T.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Carr, Department of Haematology, Guy’s and St Thomas’ Hospital, London, SE1 9RT, United Kingdom; e-mail: robtcarr@gmail.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal