Key Points
The transcription factor NF-E2 is mislocalized in patients with primary myelofibrosis.
Immunohistochemical staining for NF-E2 distinguishes essential thrombocythemia from primary myelofibrosis.
Abstract
The World Health Organization (WHO) classification of myeloproliferative neoplasms (MPNs) comprises several entities including essential thrombocythemia (ET); primary myelofibrosis (PMF); and MPN, unclassifiable (MPN,U). Differential diagnosis between ET and early, prefibrotic PMF can be challenging but is critical because clinical course and outcome vary considerably between these entities. We have previously shown that the transcription factor nuclear factor erythroid 2 (NF-E2) is aberrantly expressed in MPN patients. Here we demonstrate that NF-E2 is mislocalized in PMF cells and that aberrant NF-E2 localization discriminates statistically highly significantly between ET and PMF. A threshold of 20% nuclear NF-E2 staining was cross-validated by “.682+ bootstrapping.” Moreover, this cutoff correctly classifies diagnostic bone marrow biopsies of MPN,U patients specified upon follow-up as ET or PMF with 92% accuracy. Because interobserver concordance between independent pathologists was high (Spearman’s rank correlation coefficient, 0.727), we propose that quantitative NF-E2 immunohistochemistry represents a diagnostic tool that can reliably support a differential diagnosis between ET and PMF.
Introduction
In 1951, Dr William Dameshek first classified a group of clinically interrelated disorders, among them polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), naming them the myeloproliferative syndromes.1 The diagnostic criteria for these diseases, renamed myeloproliferative neoplasms (MPNs), were last revised by the World Health Organization (WHO) in 2008.2
Despite the discovery of an activating point mutation in the JAK2 kinase (JAK2V617F) in the majority of MPN patients,3-6 the differential diagnosis between ET and PMF can remain challenging.7 Particular in the early stages, both the clinical presentation and the histopathological appearance of ET and PMF can be similar, a problem that has sparked lively discussion on distinguishing diagnostic criteria, including those of the WHO classification.8-10 In addition, the WHO classification has been criticized for relying on histopathology, which may be subject to a high degree of interobserver variability.8,9 However, because of the large difference in clinical course and outcome between ET and PMF, accurate classification and diagnosis of these entities is essential.10-13
We have previously reported that expression of the transcription factor nuclear factor erythroid 2 (NF-E2) is aberrantly elevated in patients with MPN.14 In ET and PMF, NF-E2 overexpression is independent of the presence or absence of the JAK2V617F mutation.15 Moreover, in a murine model, elevated NF-E2 levels cause an MPN phenotype.16
Here we test the hypothesis that immunohistochemical staining for NF-E2 can be used to distinguish ET from PMF and that this distinction can aid in the classification of MPN, unclassifiable (MPN,U) patients.
Patients, materials, and methods
Patients and bone marrow biopsies
A cohort of 163 bone marrow biopsies, which had been referred to and evaluated in the Institute of Pathology, Medical Center Freiburg, between 2001 and 2010, was analyzed (Table 1). The first set consisted of 72 cases: 14 healthy controls (HC) obtained from patients with lymphoma biopsied for staging, which showed neither bone marrow infiltration nor complete blood count abnormalities; 10 patients with reactive thrombocytosis (RT); 41 patients with essential thrombocythemia (ET); 39 patients with PMF, with the fibrosis grades MF-0 (n = 10), MF-1 (n = 19), MF-2 (n = 8), and MF-3 (n = 2); and 33 patients with PV. The study was approved by the local internal review board (Albert-Ludwigs University, Freiburg, Germany). The study was conducted in accordance with the Declaration of Helsinki.
Clinical data at the time of the initial biopsies
| Group . | Number of patients . | WBC, median (range) . | PLT, median (range) . | HB, median (range) . | LDH, median (range) . | Cases with splenomegaly . | Cases with JAK2V617F (%) . |
|---|---|---|---|---|---|---|---|
| HC | 14 | 6.9 (3.7-10.0) | 242 (227-300) | 14 (12.0-14.6) | ns | ns | ns |
| RT | 10 | 9.1 (6.9-19.7) | 780 (503-1078) | 10 (8.9-13.5) | ns | ns | ns |
| PV | 33 | 11.2 (5.9-20.0) | 442 (266-897) | 19.0 (16.8-23.2) | 309 (174-689) | 13/18* | 100 |
| ET | 41 | 9.2 (1.6-18.4) | 801 (450-7400) | 14.2 (11.7-16.6) | 251 (181-339) | 4/15* | 51 |
| PMF | 39 | 10.5 (2.4-40.0) | 525 (15-1811) | 12.1 (6.7-15.5) | 447 (92-1455) | 13/18* | 59 |
| MPN,U-ET | 10 | 9.2 (5.1-12.9) | 809 (600-1696) | 15 (13.7-16.5) | 201 (142-275) | 2/9* | ns |
| MPN,U-PMF | 9 | 11.5 (8.9-18.4) | 790 (500-1056) | 14.0 (8.6-16.0) | 293 (184-401) | 5/8* | ns |
| ET-PMF | 7 | 9.0 (3.5-16.7) | 903 (602-1959) | 12.0 (5.5-14.0) | 364 (230-489) | 3/7* | ns |
| Group . | Number of patients . | WBC, median (range) . | PLT, median (range) . | HB, median (range) . | LDH, median (range) . | Cases with splenomegaly . | Cases with JAK2V617F (%) . |
|---|---|---|---|---|---|---|---|
| HC | 14 | 6.9 (3.7-10.0) | 242 (227-300) | 14 (12.0-14.6) | ns | ns | ns |
| RT | 10 | 9.1 (6.9-19.7) | 780 (503-1078) | 10 (8.9-13.5) | ns | ns | ns |
| PV | 33 | 11.2 (5.9-20.0) | 442 (266-897) | 19.0 (16.8-23.2) | 309 (174-689) | 13/18* | 100 |
| ET | 41 | 9.2 (1.6-18.4) | 801 (450-7400) | 14.2 (11.7-16.6) | 251 (181-339) | 4/15* | 51 |
| PMF | 39 | 10.5 (2.4-40.0) | 525 (15-1811) | 12.1 (6.7-15.5) | 447 (92-1455) | 13/18* | 59 |
| MPN,U-ET | 10 | 9.2 (5.1-12.9) | 809 (600-1696) | 15 (13.7-16.5) | 201 (142-275) | 2/9* | ns |
| MPN,U-PMF | 9 | 11.5 (8.9-18.4) | 790 (500-1056) | 14.0 (8.6-16.0) | 293 (184-401) | 5/8* | ns |
| ET-PMF | 7 | 9.0 (3.5-16.7) | 903 (602-1959) | 12.0 (5.5-14.0) | 364 (230-489) | 3/7* | ns |
WBC, white blood cell count ×106 per µL; PLT, platelet count ×106 per µL; HB, hemoglobin in g/dL; LDH, lactate dehydrogenase in U/L; ns, not specified.
Data were not available for the remainder of the patients.
The second set consisted of 26 patients who presented with thrombocythemia. Of these, 19 patients were initially diagnosed as MPN,U. The WHO defines these as cases that show definite clinical, laboratory, and morphologic features of an MPN but who fail to meet the criteria for any of the specific MPN entities.2,17 By follow-up biopsy, 1 to 9 years later, and by clinical course, these 19 cases were subsequently diagnosed as either ET (n = 10; MPN,U-ET) or PMF (n = 9; MPN,U-PMF).
In addition, the second set contained 7 patients (here called ET-PMF) initially interpreted by us as ET, who were, however, found to satisfy the criteria for PMF including characteristic histologic atypia in the follow-up biopsy 1 to 8 years later. These are nonetheless not examples of ET in which fibrosis developed, so-called “post–ET-MF” by WHO definition, because this definition of post–ET-MF requires a degree of fibrosis grade 2, which these patients did not show. In addition, these patients transformed to PMF within a median of 1 year after the initial diagnosis, 6 of these 7 patients transformed within 2 years after the diagnosis. When it occurs, post–ET-MF takes much more time to develop, patients transforming with a median of 8 to 9 years after diagnosis (range, 3.6-20.2).18,19
All cases were diagnosed according to the WHO criteria.2,17 To achieve pathological diagnoses by consensus of multiple observers, 3 hematopathologists, each blinded to both the initial diagnosis and the diagnoses made by his or her colleagues, re-reviewed all biopsies, which were stained for chloracetate-esterase (CAE), Giemsa, hematoxylin and eosin (H&E), and reticulin (Gomorri stain). Only those patients were chosen for inclusion in this study, for which all 3 pathologists independently arrived at the same diagnosis according to WHO criteria, and for which this diagnosis was in agreement with the initial diagnosis.
Importantly, this panel of pathologists arrived at the same diagnosis and matched the initial diagnosis in 113/125 (90.4%) of cases. Only 12 of an initially available pool of 125 biopsies (9.6%) had to be excluded because of interpathologist disagreement. In addition to the histologies, the pathologists had the following clinical information: complete blood count for all patients; LDH for the majority of patients; splenomegaly for a subset of patients, mainly those with suspected PMF, JAK2V617F for all suspected PV cases; and the majority of suspected ET and PMF cases.
The biopsies used for NF-E2 staining were obtained at initial diagnosis and were therapy-naïve.
NF-E2 and CD71 immunohistochemistry
Bone marrow biopsies were either fixed in 4% buffered formalin (FA) or in calcium-glutaraldehyde-formaldehyde (CGF) (0.1 mol/L calcium acetate, 1.1 vol % formaldehyde, and 0.5 vol % glutaraldehyde), as described.20 After fixation, all biopsies were subjected to decalcification in a mixture of 10% ethylenediaminetetraacetic acid disodium salt (Serva) and 3.3% tris-(hydroxymethyl) aminomethane (AppliChem) in dd H2O at a pH of 7.0 to 7.2 overnight or for 2 days at room temperature, as described,20 and then embedded in paraffin. Decalcification for either 1 or 2 days yielded identical staining results (data not shown).
Serial 3-µm sections were deparaffinized in xylene and graded alcohols, followed by specific antigen retrieval in target retrieval solution (pH 9) in a steamer (Dako, Glostrup, Denmark; 20 minutes for CGF-fixed biopsies and 4-6 minutes for FA-fixed biopsies, depending on the NF-E2 antibody lot). After incubation with 1 of 2 primary antibodies against NF-E2 for 1 hour at room temperature (NF-E2, polyclonal rabbit [Sigma Aldrich], diluted 1:50 for CGF-fixed biopsies and 1:200 for FA fixed biopsies or anti-NF-E2, rabbit polyclonal 1089, diluted 1:100, raised against amino acids 133-147 of the NF-E2 protein21 ), staining was detected with the Dako Real Detection System (Dako; antibody by Sigma) or the Dako EnVision FLEX Visualization System (Dako; antibody 1089). The sections were counterstained with hematoxylin (Dako) and mounted. To exclude unspecific staining, negative controls were prepared by replacing NF-E2 with an immunoglobulin isotype control (ChromPure Rabbit IgG; Jackson ImmunoResearch).
For double staining with CD71, NF-E2 staining and detection were followed by a peroxidase block. Subsequently, an anti-CD71 antibody was applied for 20 minutes at room temperature (DCS, mouse monoclonal, clone H68.4; ready to use). Staining was detected with the Dako EnVision FLEX Visualization System (Dako).
NF-E2–specific staining was evaluated in 300 erythroid cells, 100 in each of 3 random high-power fields (600× original magnification) per case. Every biopsy was evaluated independently by 2 pathologists, both blinded to the diagnosis. The percentage (mean ± SD) of nuclear-positive, cytoplasmic-positive, and negative erythroid cells as a proportion of all nucleated erythropoietic precursor cells was calculated for each biopsy. Cells that were both nuclear- and cytoplasmic-positive were regarded as nuclear-positive.
Statistical analysis
Statistical analyses were performed after the data of both observers had been averaged. A 2-tailed Wilcoxon test was used to determine whether a significant (P < .05) difference existed between the 2 groups. When comparing more than 2 groups, a Kruskal-Wallis 1-way analysis of variance on ranks was used.
A Spearman’s rank-order correlation was run to determine the concordance between the 2 pathologists’ nuclear NF-E2 quantification.
SPSS Software 18.0.2 (IBM Corporation, Armonk, NY) was used for analysis.
Threshold calculation and cross-validation by .632+ bootstrapping
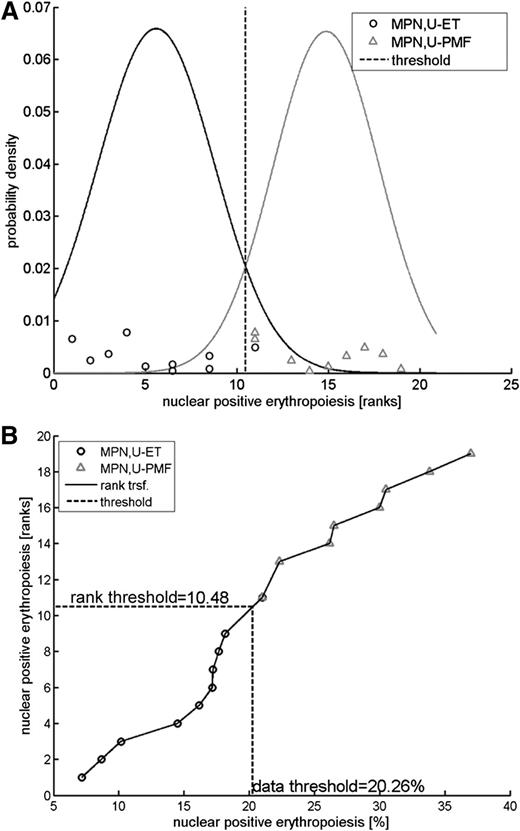
For threshold calculation, a classification was performed after rank transformation of the measurements using a Naïve Bayes classifier as shown in Figure 1. A threshold of 20.3% was calculated to optimally discriminate between MPN,U-ET and MPN,U-PMF patients.
Rank transformation and threshold calculation. Rank transformations constitute an efficient strategy to perform robust analyses with respect to distributional assumptions.22 (A) Posterior probability densities for MPN,U-ET and MPN,U-MPF patients fitted to the measurements after rank transformation. A rank transformation of the data was applied to perform the classification analysis. The posterior probability densities are plotted for both MPN,U-ET and MPN,U-PMF patients. The threshold of 10.48 for the ranks is obtained by determining the rank with equal posterior probabilities. (B) Transformation. The threshold value for the ranks (10.48) was translated to the measurement scale. Ranks are plotted in a vertical direction; the horizontal axis denotes the measurements as percent of nuclear-positive cells. The threshold 10.48 for ranks (vertical axis) corresponds to 20.26% nuclear-positive cells (horizontal axis).
Rank transformation and threshold calculation. Rank transformations constitute an efficient strategy to perform robust analyses with respect to distributional assumptions.22 (A) Posterior probability densities for MPN,U-ET and MPN,U-MPF patients fitted to the measurements after rank transformation. A rank transformation of the data was applied to perform the classification analysis. The posterior probability densities are plotted for both MPN,U-ET and MPN,U-PMF patients. The threshold of 10.48 for the ranks is obtained by determining the rank with equal posterior probabilities. (B) Transformation. The threshold value for the ranks (10.48) was translated to the measurement scale. Ranks are plotted in a vertical direction; the horizontal axis denotes the measurements as percent of nuclear-positive cells. The threshold 10.48 for ranks (vertical axis) corresponds to 20.26% nuclear-positive cells (horizontal axis).
The classification error rate was derived by cross-validation using bootstrapping, a method that repeatedly divides the data into training and test sets. For this analysis, 10 000 bootstrap data sets were drawn of the same sample size as the experimental data set (10 MPN,U-ET and 9 MPN,U-PMF). The bootstrap sets are randomly selected from the experimental data set with replacement. For each of the 10 000 bootstrap data sets, the rank transformation was applied and the classification threshold was calculated and subsequently evaluated on how often the classifier correctly predicts for out-of-sample. For the 10 000 realizations, the average out-of-sample classification error was 9.7%. Because this number is known to overestimate the classification error, the so-called .632+ estimator23 was applied for adjustment, such that the out-of-sample error is reasonably weighted with the in-sample classification error (5.3%). In our analysis, a weight of 0.66 was derived for the .632+ estimator, yielding an expected classification error of 8.2% for validation measurements.
Results
Subcellular localization of NF-E2 during erythroid maturation
The transcription factor NF-E2 is known to be expressed in several hematopoietic lineages.24 However, its subcellular localization during distinct stages of erythroid differentiation is unknown. We therefore performed immunohistochemistry of healthy bone marrow biopsies and scored nuclear or cytoplasmic NF-E2 localization in various stages of erythroid maturation. All 14 biopsies were scored independently by 2 pathologists.
In healthy bone marrow, megakaryocytes were predominantly NF-E2–positive in the cytoplasm or the nucleus, or in both compartments. In addition, cells of the myeloid lineage were weakly positive for NF-E2. Again both nuclear and cytoplasmatic staining was observed. Lymphocytes did not stain for NF-E2.
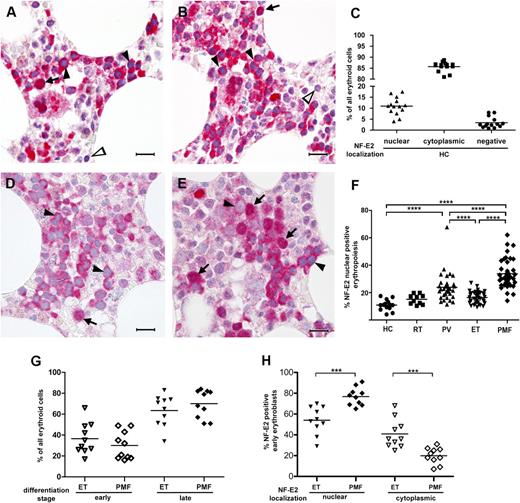
In early erythropoietic cells, NF-E2 showed nuclear staining (Figure 2A-B, arrows), whereas, unexpectedly, in later nucleated erythroid stages it was found almost exclusively in the cytoplasm (Figure 2A-B, filled arrowheads). Surprisingly for a transcription factor, on average only 10.9% (standard deviation, SD ±3.8) of all erythropoietic cells, mainly the early erythroblasts, showed a nuclear NF-E2 positivity (Figure 2C), whereas the vast majority, on average 85.7% (±2.2%), stained cytoplasmically (Figure 2C). Very few erythroid cells (an average of 3.4% ± 2.5%) were completely negative for NF-E2 (Figure 2A-C). This is the first description of strong cytoplasmic NF-E2 expression in more mature erythropoietic cells and raises the possibility that the NF-E2 protein may fulfill additional, previously unrecognized functions in the cytoplasm.
Immunohistochemistry of NF-E2 in HCs, RT, ET, PV, and PMF patients. (A-B represent HCs; D represents essential thrombocythemia; E represents PMF) Bone marrow biopsies were stained with an antibody against NF-E2 and counterstained with hematoxylin (original magnification ×1000; the bar indicates 20 µm). Arrows point to erythropoietic cells with nuclear NF-E2 staining, filled arrow heads indicate cytoplasmic NF-E2 staining, and open arrowheads mark cells negative for NF-E2 staining. (C,F-H) One hundred erythropoietic cells in each of 3 high-power fields per bone marrow biopsy (300 erythroid cells total) were evaluated for NF-E2 subcellular localization and/or differentiation stage. (C) Quantitative analysis of NF-E2 subcellular localization in HCs. Shown is the percentage of nuclear or cytoplasmic NF-E2–positive erythroid cells, respectively, and NF-E2–negative cells as a proportion of all erythroid precursors. (F) Quantitative analysis of nuclear NF-E2 positivity in erythroid cells in HC RT, and MPN patients. Shown is the percentage of nuclear NF-E2–positive erythroid cells as a proportion of all erythroid precursors. ****P < .0001 by 2-tailed Wilcoxon test. (G) Proportion of early and late erythroblasts of all erythroid cells in ET and PMF patients. Shown is the percentage of early and late erythroblasts as a proportion of all erythroid cells in ET and PMF patients. An early erythroblast was defined on a CAE stain as a CAE-negative erythroid cell with a small cytoplasm, a large nucleus (1.5-2.5–fold of the diameter of an erythrocyte), and 1 or 2 prominent nucleoli. A late erythroblast was defined as a CAE-negative cell, with abundant cytoplasm, frequently polygonal in shape, and with a round nucleus with dense chromatin. (H) Proportion of NF-E2 nuclear or cytoplasmic positive early erythroblasts in ET and PMF patients. Shown is the percentage of nuclear or cytoplasmic NF-E2–positive cells, as indicated, in early erythroid precursors. ***P < .001 by 2-tailed Wilcoxon test.
Immunohistochemistry of NF-E2 in HCs, RT, ET, PV, and PMF patients. (A-B represent HCs; D represents essential thrombocythemia; E represents PMF) Bone marrow biopsies were stained with an antibody against NF-E2 and counterstained with hematoxylin (original magnification ×1000; the bar indicates 20 µm). Arrows point to erythropoietic cells with nuclear NF-E2 staining, filled arrow heads indicate cytoplasmic NF-E2 staining, and open arrowheads mark cells negative for NF-E2 staining. (C,F-H) One hundred erythropoietic cells in each of 3 high-power fields per bone marrow biopsy (300 erythroid cells total) were evaluated for NF-E2 subcellular localization and/or differentiation stage. (C) Quantitative analysis of NF-E2 subcellular localization in HCs. Shown is the percentage of nuclear or cytoplasmic NF-E2–positive erythroid cells, respectively, and NF-E2–negative cells as a proportion of all erythroid precursors. (F) Quantitative analysis of nuclear NF-E2 positivity in erythroid cells in HC RT, and MPN patients. Shown is the percentage of nuclear NF-E2–positive erythroid cells as a proportion of all erythroid precursors. ****P < .0001 by 2-tailed Wilcoxon test. (G) Proportion of early and late erythroblasts of all erythroid cells in ET and PMF patients. Shown is the percentage of early and late erythroblasts as a proportion of all erythroid cells in ET and PMF patients. An early erythroblast was defined on a CAE stain as a CAE-negative erythroid cell with a small cytoplasm, a large nucleus (1.5-2.5–fold of the diameter of an erythrocyte), and 1 or 2 prominent nucleoli. A late erythroblast was defined as a CAE-negative cell, with abundant cytoplasm, frequently polygonal in shape, and with a round nucleus with dense chromatin. (H) Proportion of NF-E2 nuclear or cytoplasmic positive early erythroblasts in ET and PMF patients. Shown is the percentage of nuclear or cytoplasmic NF-E2–positive cells, as indicated, in early erythroid precursors. ***P < .001 by 2-tailed Wilcoxon test.
NF-E2 is mislocalized in MPN biopsies and shows increased nuclear staining
Because of the documented NF-E2 overexpression in MPN patients, we hypothesized that NF-E2 staining or subcellular localization may differ between MPN patients and HCs. We therefore assembled a cohort of MPN bone marrow biopsies, diagnosed according to the WHO criteria, consisting of 33 PV, 41 ET, and 39 PMF cases. In addition to fulfilling the WHO classification criteria, most cases had undergone follow-up biopsies at least 1 year after the initial diagnosis. At this time, the MPN entity was confirmed by both pathological analysis and clinical course. The biopsies obtained at the time of diagnosis were stained for NF-E2 and again scored for subcellular localization in erythroid cells. The 2 independently scoring pathologists were blinded to the diagnoses.
As in HCs, ET cases showed a low proportion of nuclear positivity for NF-E2 (16.3% ± 4.1% of all erythroid cells; Figure 2D,F). Patients with RT, which serve as an important control, likewise showed low numbers of NF-E2 nuclear-positive erythroid cells (15.1 ± 3.4%, Figure 1F). In contrast, PV cases showed a slightly higher proportion of nuclear positivity in erythroid cells (23.9% ± 10%), and this difference reached statistical significance (Figure 2F; P < .0001 vs both HC and ET). Most striking is the stark and statistically highly significant increase in nuclear NF-E2 staining observed in PMF patients (33.7% ± 10.7%: P < .0001 vs PV, ET, and HC each; Figure 2E-F). This marked increase in the proportion of NF-E2 nuclear-positive erythroid cells was apparent even in patients with grade 0 or 1 fibrosis, suggesting that increased nuclear staining of the transcription factor is inherent to disease development, rather than being a feature of disease progression.
To verify that the cells scored for NF-E2 staining were indeed erythroblasts, we conducted double stainings with NF-E2 and CD71, a marker of early erythroid cells. These data were quantitated and are shown in supplemental Figure 2. They reveal that, first, more than 95% of all cells that stain nuclear for NF-E2 in the bone marrow are CD71-positive erythroblasts (supplemental Figure 2C) and, second, that counting only CD71 positive cells (ie, cells that have been identified as erythroid because of a surface marker), PMF patients show a statistically highly significant increase in nuclear NF-E2 staining (supplemental Figure 2D).
We wished to confirm these differences in NF-E2 staining with a second, alternative antibody generated in our laboratory and raised against a different NF-E2 peptide than the antibody that was initially used. Cytoplasmic and nuclear NF-E2 staining in erythroid cells was again quantitated in ET and PMF patients. As with the first antibody used, a statistically highly significant difference between ET and PMF patients was observed (supplemental Figure 1). This second antibody therefore confirms both the cytoplasmic staining of NF-E2 in mature erythroid cells and the mislocalization of NF-E2 in PMF patients.
Because early erythropoiesis shows nuclear NF-E2 staining, whereas more mature erythroid cells display cytoplasmic NF-E2 (Figure 2A-C), the increase in NF-E2 nuclear staining observed in PMF could simply result from the presence of increased numbers of early erythroblasts in PMF bone marrow. We therefore quantified the percentage of early and late erythropoietic cells in ET and PMF patients (Figure 2G). There was no difference in the percentage of early and late erythropoietic cells between ET and PMF. Rather, a significantly higher percentage of early erythroblasts showed nuclear NF-E2 staining in PMF compared with ET, demonstrating that the NF-E2 protein is mislocalized in PMF (Figure 2H). Aberrant localization could occur by abnormal retention in the nucleus, by insufficient cytoplasmic export, or by increased reimport into this organelle.
Because the percentage of nuclear NF-E2 positivity in erythroid cells is highly significantly different between PMF and ET biopsies (P < .0001, Figure 2F), we proposed that this staining could be used to discriminate between the early, prefibrotic stage of PMF and ET, entities that are often challenging to differentiate diagnostically.
Quantitative NF-E2 immunohistochemistry discriminates between early, prefibrotic PMF and ET
To test the hypothesis that NF-E2 staining can discriminate between early, prefibrotic PMF and ET, we analyzed a second MPN cohort. This cohort again consisted entirely of cases that were diagnosed according to the WHO criteria and had both follow-up biopsies and clinical data available for all patients. The WHO classification recognizes an entity termed “MPN-Unclassifiable (MPN,U)” for MPN patients who do not clearly fulfill the diagnostic criteria for PV, ET, or PMF. Upon follow-up, if these patients then fulfill the WHO criteria for another MPN entity, they may be reclassified. Our second cohort consisted of 19 MPN,U patients, 10 of whom were reclassified as ET upon follow-up (MPN,U-ET, Figure 3A) and 9 who were reclassified as PMF upon follow-up (MPN,U-PMF, Figure 3B). In addition, we included 7 patients whose initial diagnosis of ET was revised to PMF upon follow-up (ET-PMF, Figure 3C).
Immunohistochemistry of NF-E2 in MPN,U, ET, and PMF patients. Bone marrow biopsies were stained with an antibody against NF-E2 and counterstained with hematoxylin (original magnification ×1000, bar indicates 20 µm). Arrows point to erythropoietic cells with nuclear NF-E2 staining and filled arrowheads indicate cytoplasmic NF-E2 staining. (A) MPN,U later reclassified as ET. (B) MPN,U later reclassified as PMF. (C) ET later reclassified as PMF. (D) Quantitative analysis of NF-E2 immunohistochemistry in MPN,U patients. One hundred erythropoietic cells in each of 3 high-power fields per bone marrow biopsy were evaluated (300 erythroid cells in total). Shown is the percentage of nuclear NF-E2–positive erythroid cells as a proportion of all erythroid precursors. Data for ET and PMF are presented as in Figure 2F; **P < .01, ****P < .0001 by Wilcoxon test.
Immunohistochemistry of NF-E2 in MPN,U, ET, and PMF patients. Bone marrow biopsies were stained with an antibody against NF-E2 and counterstained with hematoxylin (original magnification ×1000, bar indicates 20 µm). Arrows point to erythropoietic cells with nuclear NF-E2 staining and filled arrowheads indicate cytoplasmic NF-E2 staining. (A) MPN,U later reclassified as ET. (B) MPN,U later reclassified as PMF. (C) ET later reclassified as PMF. (D) Quantitative analysis of NF-E2 immunohistochemistry in MPN,U patients. One hundred erythropoietic cells in each of 3 high-power fields per bone marrow biopsy were evaluated (300 erythroid cells in total). Shown is the percentage of nuclear NF-E2–positive erythroid cells as a proportion of all erythroid precursors. Data for ET and PMF are presented as in Figure 2F; **P < .01, ****P < .0001 by Wilcoxon test.
The proportion of nuclear NF-E2–positive erythroid cells in the initial biopsies of MPN,U cases later diagnosed as ET was as low as that observed in ET patients (14.8% ± 4.3%, Figure 3D). In contrast, NF-E2 nuclear staining in initial biopsies of MPN,U cases later reclassified as PMF (27.6% ± 5.4%) was statistically highly significantly elevated compared with either ET or MPN,U-ET cases (both P < .0001; Figure 3D) and similar to that of PMF cases (Figure 3D). Likewise, biopsies of patients initially diagnosed as ET who were later reclassified as PMF cases revealed a high proportion of NF-E2 nuclear-positive erythroid cells in their initial biopsies (27.6% ± 6.7%), comparable with that of PMF cases (Figure 3D). ET-PMF cases differ highly significantly from both ET (P < .0001) and MPN,U-ET cases (P < .01) (Figure 3D).
We therefore propose that NF-E2 immunohistochemistry allows a discrimination of MPN patients with ET from those with early, prefibrotic PMF, which is especially important for those patients whose clinical presentation and bone marrow morphology do not allow this differential diagnosis. The threshold for NF-E2 nuclear positivity was calculated to be 20%, with samples showing more nuclear NF-E2–positive erythropoiesis that can be classified as PMF and those showing less nuclear NF-E2–positive erythropoiesis classified as ET (Figure 1A). To validate this threshold, we performed a “.682+ bootstrapping” cross-validation. The 10 000 bootstrap data sets generated predict a correct distinction between ET and prefibrotic PMF in 92% of cases, hence an error rate of 8% using 20% nuclear NF-E2 as a cut off (Figure 1B).
Morphologic analysis has been criticized, especially in the MPN field, for being difficult to apply in daily practice because it may be subject to considerable interobserver variability.9,25 We therefore calculated the interobserver variability between the 2 pathologists, who scored the biopsies blinded both to the diagnosis and the results of the other researcher. Spearman’s rank correlation coefficient of 0.727 demonstrates an extremely high interobserver consistency for all 163 cases (P < .001).
Discussion
Despite the rising number of molecular aberrations detected in MPN patients, a distinction among the 3 related entities, especially between the early, prefibrotic phase of PMF and ET remains difficult and relies mainly on histology and clinical diagnosis. The reason is that none of the mutations detected in MPN patients are exclusive to any 1 entity.11 In addition, many of the mutations are found in a small subset of patients (5%-10%) and the costs of searching for 10 possible mutations in any 1 patient are prohibitive given the current technology. Therefore, in patients with isolated thrombocytosis, histology remains one of the most important tools for distinguishing ET from prefibrotic PMF.
However, it has been criticized that histology alone is insufficient in its discriminatory power and interobserver variability remains a concern.25 Additional tools for distinction of ET from prefibrotic PMF would clearly be beneficial not only for diagnosis but also for the interpretation of clinical trial results. For example, the PT1 trial, which investigated the use of hydroxyurea and anagrelide in ET,26 has been criticized for including prefibrotic PMF patients in its cohort.10,27,28
Here we describe a simple, highly reproducible immunohistochemical stain, which demonstrated a low interobserver variability and a high degree of accuracy. Interestingly, the proportion of nuclear NF-E2 staining in PMF patients is already significantly elevated at diagnosis and remains stable during follow-up (supplemental Figure 3). Our data indicate that analysis of the proportion of nuclear NF-E2–positive erythroid cells represents a viable diagnostic tool that can add a highly reliable support to reaching a differential diagnosis between ET and PMF.
In summary, quantitative NF-E2 immunohistochemistry of bone marrow biopsies of MPN patients presenting with thrombocytosis can help to distinguish ET from early, prefibrotic PMF, with important consequences for both therapeutic decisions and prognostic implications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Theresa Lowka and Katja Thurig for technical assistance and Dr Heiko Becker and Jonas Jutzi for critical review of the manuscript.
This work was supported by grants from the Medical Faculty of the University of Freiburg (AUM843/11) as well as from the Deutsche Gesellschaft für Pathologie, DGP (K.A.), as well as by grants from the National Cancer Institute (PO1 CA108671) and the Deutsche Forschungsgemeinschaft, DFG (Pa 611/6-1) (H.L.P.).
Authorship
Contribution: K.A. designed experiments, conducted the experiments, analyzed the data, and wrote the manuscript; A.V.F., A.M.M., J.P.M., C.K., J.T., and D.H. analyzed data; M.W. analyzed data and critically reviewed the manuscript; and H.L.P. designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heike L. Pahl, Division of Molecular Hematology, University Medical Center Freiburg, Center for Clinical Research, Breisacher Strasse 66, 79106 Freiburg, Germany; e-mail: heike.pahl@uniklinik-freiburg.de.