Abstract
Cancer-associated venous thrombosis is a common condition, although the reported incidence varies widely between studies depending on patient population, start and duration of follow-up, and the method of detecting and reporting thrombotic events. Furthermore, as cancer is a heterogeneous disease, the risk of venous thrombosis depends on cancer types and stages, treatment measures, and patient-related factors. In general, cancer patients with venous thrombosis do not fare well and have an increased mortality compared with cancer patients without. This may be explained by the more aggressive type of malignancies associated with this condition. It is hypothesized that thromboprophylaxis in cancer patients might improve prognosis and quality of life by preventing thrombotic events. However, anticoagulant treatment leads to increased bleeding, particularly in this patient group, so in case of proven benefit of thromboprophylaxis, only patients with a high risk of venous thrombosis should be considered. This review describes the literature on incidence of and risk factors for cancer-associated venous thrombosis, with the aim to provide a basis for identification of high-risk patients and for further development and refinement of prediction models. Furthermore, knowledge on risk factors for cancer-related venous thrombosis may enhance the understanding of the pathophysiology of thrombosis in these patients.
Introduction
In 1865, Armand Trousseau, a French physician, was one of the first to describe an association between thrombosis and cancer. Not many know the association had already been reported earlier in 1823 by Jean Baptiste Bouillaud.1,2 Perhaps because of the irony of Trousseau diagnosing the condition on himself and dying from it in 1867, the condition was later called Trousseau syndrome. Since then, many studies have confirmed the association between cancer and venous thrombosis and demonstrated that the incidence of venous thrombosis in cancer patients is high, that it has risen over the last decades, and that cancer patients with venous thrombosis do not fare well. It is hypothesized that thromboprophylaxis targeted at cancer patients with a particular high risk of thrombosis might improve their prognosis. Therefore, a need exists to identify such patients, which is not easy because cancer is a heterogeneous disease, and the risk of venous thrombosis depends on the interaction between tumor cells, the hemostatic system, and characteristics of the patient. Furthermore, identification of risk factors for cancer-related venous thrombosis will help to improve understanding of the pathophysiology of thrombosis in cancer patients. Thus, even 150 years after Trousseau died, there is still a need to study the epidemiology of venous thrombosis and cancer in detail.
Incidence of venous thrombosis in cancer patients
It is estimated consistently that ∼20% to 30% of all first venous thromboembolic events are cancer associated (Table 1).3-9 In a population-based, nested case-control study within the Olmsted County population (Minnesota), 625 residents with incident deep vein thrombosis (DVT) or pulmonary embolism (PE) were matched by age and gender to 625 unaffected residents. A population attributable risk (the percentage of all cases of a disease in a population that can be attributed to a risk factor) was calculated and reported to be 18% (95% confidence interval [CI]: 13.4-22.6) for an active malignancy.5 White and coworkers used the California discharge data set to identify a cohort of 21 002 patients hospitalized with incident venous thrombosis in 1996. Of these patients, again ∼20% (4368) were reported to have cancer-associated venous thrombosis.9 In a third study, medical records of residents from the Worcester metropolitan area were obtained for a total of 1399 subjects with a confirmed episode of venous thrombosis. Of these patients, 29% had a recent or active malignant neoplasm.8 In a more recent registry, the Registro Informatizado de Enfermedad Trombo Embólica (RIETE) registry, which included >35 000 consecutive symptomatic venous thrombosis patients from 2001 to 2011, active cancer was reported in 6075 patients (17%).4 Last, the Tromsø study is a population-based prospective follow-up study of >26 000 subjects. Participants were followed for venous thrombosis from 1994 to 2007. Of 462 patients with a first-ever venous thrombosis event, 106 had an active cancer (23%).3
Incidences and risk factors for venous thrombosis as discussed in the review
| Topic . | Study population . | Study design . | Number of patients . | Effect estimate . | Reference . |
|---|---|---|---|---|---|
| Proportion of cancer-associated VT cases | Olmsted county population | Nested case-control | 625/625 | 18% (PAR) | 5 |
| California Discharge DataSet | Cohort | 21 002 | 21% | 9 | |
| Worcester metropolitan area, outpatient setting | Cohort | 1399 | 29% | 8 | |
| RIETE Registry | Cohort | 35 539 | 17% | 4 | |
| Tromsø Study | Cohort | 462 | 23% | 3 | |
| RR of VT for cancer vs no cancer | MEGA study | Case-control | 2131/3220 | OR 6.7 (95% CI; 5.2-8.6) | 10 |
| Olmsted county population | Nested case-control | 625/625 | OR 4.1 (95% CI; 1.9-8.5) | 12 | |
| Linked United Kingdom databases | Cohort | 82 203/577 207 | HR 4.7 (95% CI; 4.5-4.9) | 13 | |
| Danish population-based registries | Cohort | 57 591/287 476 | HR 4.7 (95% CI; 4.3-5.1) | 11 | |
| Absolute risk of VT in cancer patients | Linkage of California Cancer Registry and California Discharge Dataset | Cohort | 235 149 | 1.6% within 2 y | 14 |
| Referred patients with solid tumors | Cohort | 1041 | 7.8% (median follow-up 26 mo) | 15 | |
| CATS study | Cohort | 840 | 8% within 1 y | 16 | |
| 38 papers on cohorts with cancer patients | Meta-analysis | NA | 13/1000 PY (95% CI; 7-23) for average-risk patients | 17 | |
| 68/1000 PY (95% CI; 48-96) for high-risk patients | 17 | ||||
| Linked United Kingdom databases | Cohort | 82 203 | 14/1000 PY (95% CI; 13-14) | 13 | |
| Incidence of VT in cancer patients over time | US National Hospital Discharge Survey | Cohort | 40 787 000 | 1.5% in 1989; 3.5% in 1999 | 19 |
| Discharge Database from University HealthSystem Consortium | Cohort | 1 015 598 | ∼3.5% in 1995; ∼4.5% in 2002 | 18 | |
| Linked United Kingdom databases | Cohort | 82 203 | 10.3/1000 PY in 1997; 19/1000 PY in 2006 | 13 | |
| Risk factors for VT in cancer patients | |||||
| Type of cancer | 38 papers on cohorts with cancer patients | Meta-analysis | NA | Pancreatic cancer: ∼110/1000 PY | 17 |
| Brain cancer: ∼80/1000 PY | |||||
| Lung cancer: ∼45/1000 PY | |||||
| Haematologic cancer: ∼40/1000 PY | |||||
| Colorectal cancer: ∼30/1000 PY | |||||
| Bone cancer: ∼30/1000 PY | |||||
| Prostate cancer: ∼10/1000 PY | |||||
| Breast cancer: ∼10/1000 PY | |||||
| Stage of cancer | Danish population-based registries | Cohort | 40 994/204 970 | HRs 2.9, 2.9, 7.5, and 17.1 for stage I, II, III, and IV cancer patients, respectively, vs general population | 11 |
| Linkage of California Cancer Registry and California Discharge Dataset | Cohort | 235 149 | HRs ranging from 1.1 to 21.5 for different types of cancer, metastatic vs localized cancer | 14 | |
| CATS study | Cohort | 740 | HR 2.0 (95% CI; 1.1-3.5) for (solid) tumor grade G3+G4 vs G1+G2 | 24 | |
| Time since cancer diagnosis | MEGA study | Case-control | 2131/3220 | OR 53.5 (95% CI; 8.6-334.3) in first 3 mo after cancer diagnosis | 10 |
| OR 14.3 (95% CI; 5.8-35.2) in 3-12 mo after cancer diagnosis | |||||
| OR 1.1 (95% CI; 0.6-2.2) > 15 y after cancer diagnosis | |||||
| Linkage of California Cancer Registry and California Discharge Dataset, colorectal cancer patients | Cohort | 68 142 | 5.0/100 PY 0-6 mo after cancer diagnosis 1.4/100 PY 6-12 mo after cancer diagnosis 0.6/100 PY 12-24 mo after cancer diagnosis | 25 | |
| Linked United Kingdom databases | Cohort | 82 203 | Median ratio 3.2 for VT risk in first 3 mo after diagnosis vs whole follow-up period, for cancer types separately | 13 | |
| Treatment | Olmsted county population | Nested case-control | 625/625 | OR 4.1 vs OR 6.5 for treatment with and without chemotherapy | 12 |
| Node-positive primary operable breast cancer patients | RCT | 353/352 | Cum. inc. of VT: 13.6% vs 2.6% for 2 y tamoxifen with vs without 6 mo additional chemotherapy | 33 | |
| Advanced gastroesophageal cancer patients | RCT | 490/474 | Cum. inc. of VT during and 30 days after chemotherapy: 12.2% for cisplatin vs 6.5% for oxaliplatin containing regimens | 34 | |
| 35 papers on trials with cancer patients | Meta-analysis | 6769 | RR 1.7 (95% CI; 1.4-2.1) for VT in cancer patients treated with red blood cell transfusions with vs without ESAs | 35 | |
| 38 papers on phase 3 trials with cancer patients | Meta-analysis | 8172 | RR 1.6 (95% CI; 1.3-1.9) for VT in cancer patients treated with red blood cell transfusions with vs without ESAs | 36 | |
| 15 Papers on trials with patients with solid tumors | Meta-analysis | 7956 | RR 1.3 (95% CI; 1.1-1.6) for VT in cancer patients treated with standard antineoplastic therapy with vs without bevacizumab | 37 | |
| Patient-related | Linkage of California Cancer Registry and California Discharge Dataset, colorectal cancer patients | Cohort | 68 142 | HR 2.0 (95% CI; 1.7-2.3) for 3 or more comorbid conditions vs no comorbidities HR 0.4 (95% CI; 0.3-0.5) for Asian/Pacific Islanders vs Caucasians | 25 |
| Discharge database of University Healthsystem Consortium | Cohort | 1 015 598 | ORs ranging from 1.4 to 1.8 for cancer patients with a comorbidity vs cancer patients without comorbidities | 18 | |
| OR 1.2 and 0.7 for patients with black and asian ethnicity respectively vs white | |||||
| MEGA study | Case-control | 2131/3220 | OR 2.2 (95% CI; 0.3-17.8) for VT in cancer patients with vs without factor V Leiden | 10 |
| Topic . | Study population . | Study design . | Number of patients . | Effect estimate . | Reference . |
|---|---|---|---|---|---|
| Proportion of cancer-associated VT cases | Olmsted county population | Nested case-control | 625/625 | 18% (PAR) | 5 |
| California Discharge DataSet | Cohort | 21 002 | 21% | 9 | |
| Worcester metropolitan area, outpatient setting | Cohort | 1399 | 29% | 8 | |
| RIETE Registry | Cohort | 35 539 | 17% | 4 | |
| Tromsø Study | Cohort | 462 | 23% | 3 | |
| RR of VT for cancer vs no cancer | MEGA study | Case-control | 2131/3220 | OR 6.7 (95% CI; 5.2-8.6) | 10 |
| Olmsted county population | Nested case-control | 625/625 | OR 4.1 (95% CI; 1.9-8.5) | 12 | |
| Linked United Kingdom databases | Cohort | 82 203/577 207 | HR 4.7 (95% CI; 4.5-4.9) | 13 | |
| Danish population-based registries | Cohort | 57 591/287 476 | HR 4.7 (95% CI; 4.3-5.1) | 11 | |
| Absolute risk of VT in cancer patients | Linkage of California Cancer Registry and California Discharge Dataset | Cohort | 235 149 | 1.6% within 2 y | 14 |
| Referred patients with solid tumors | Cohort | 1041 | 7.8% (median follow-up 26 mo) | 15 | |
| CATS study | Cohort | 840 | 8% within 1 y | 16 | |
| 38 papers on cohorts with cancer patients | Meta-analysis | NA | 13/1000 PY (95% CI; 7-23) for average-risk patients | 17 | |
| 68/1000 PY (95% CI; 48-96) for high-risk patients | 17 | ||||
| Linked United Kingdom databases | Cohort | 82 203 | 14/1000 PY (95% CI; 13-14) | 13 | |
| Incidence of VT in cancer patients over time | US National Hospital Discharge Survey | Cohort | 40 787 000 | 1.5% in 1989; 3.5% in 1999 | 19 |
| Discharge Database from University HealthSystem Consortium | Cohort | 1 015 598 | ∼3.5% in 1995; ∼4.5% in 2002 | 18 | |
| Linked United Kingdom databases | Cohort | 82 203 | 10.3/1000 PY in 1997; 19/1000 PY in 2006 | 13 | |
| Risk factors for VT in cancer patients | |||||
| Type of cancer | 38 papers on cohorts with cancer patients | Meta-analysis | NA | Pancreatic cancer: ∼110/1000 PY | 17 |
| Brain cancer: ∼80/1000 PY | |||||
| Lung cancer: ∼45/1000 PY | |||||
| Haematologic cancer: ∼40/1000 PY | |||||
| Colorectal cancer: ∼30/1000 PY | |||||
| Bone cancer: ∼30/1000 PY | |||||
| Prostate cancer: ∼10/1000 PY | |||||
| Breast cancer: ∼10/1000 PY | |||||
| Stage of cancer | Danish population-based registries | Cohort | 40 994/204 970 | HRs 2.9, 2.9, 7.5, and 17.1 for stage I, II, III, and IV cancer patients, respectively, vs general population | 11 |
| Linkage of California Cancer Registry and California Discharge Dataset | Cohort | 235 149 | HRs ranging from 1.1 to 21.5 for different types of cancer, metastatic vs localized cancer | 14 | |
| CATS study | Cohort | 740 | HR 2.0 (95% CI; 1.1-3.5) for (solid) tumor grade G3+G4 vs G1+G2 | 24 | |
| Time since cancer diagnosis | MEGA study | Case-control | 2131/3220 | OR 53.5 (95% CI; 8.6-334.3) in first 3 mo after cancer diagnosis | 10 |
| OR 14.3 (95% CI; 5.8-35.2) in 3-12 mo after cancer diagnosis | |||||
| OR 1.1 (95% CI; 0.6-2.2) > 15 y after cancer diagnosis | |||||
| Linkage of California Cancer Registry and California Discharge Dataset, colorectal cancer patients | Cohort | 68 142 | 5.0/100 PY 0-6 mo after cancer diagnosis 1.4/100 PY 6-12 mo after cancer diagnosis 0.6/100 PY 12-24 mo after cancer diagnosis | 25 | |
| Linked United Kingdom databases | Cohort | 82 203 | Median ratio 3.2 for VT risk in first 3 mo after diagnosis vs whole follow-up period, for cancer types separately | 13 | |
| Treatment | Olmsted county population | Nested case-control | 625/625 | OR 4.1 vs OR 6.5 for treatment with and without chemotherapy | 12 |
| Node-positive primary operable breast cancer patients | RCT | 353/352 | Cum. inc. of VT: 13.6% vs 2.6% for 2 y tamoxifen with vs without 6 mo additional chemotherapy | 33 | |
| Advanced gastroesophageal cancer patients | RCT | 490/474 | Cum. inc. of VT during and 30 days after chemotherapy: 12.2% for cisplatin vs 6.5% for oxaliplatin containing regimens | 34 | |
| 35 papers on trials with cancer patients | Meta-analysis | 6769 | RR 1.7 (95% CI; 1.4-2.1) for VT in cancer patients treated with red blood cell transfusions with vs without ESAs | 35 | |
| 38 papers on phase 3 trials with cancer patients | Meta-analysis | 8172 | RR 1.6 (95% CI; 1.3-1.9) for VT in cancer patients treated with red blood cell transfusions with vs without ESAs | 36 | |
| 15 Papers on trials with patients with solid tumors | Meta-analysis | 7956 | RR 1.3 (95% CI; 1.1-1.6) for VT in cancer patients treated with standard antineoplastic therapy with vs without bevacizumab | 37 | |
| Patient-related | Linkage of California Cancer Registry and California Discharge Dataset, colorectal cancer patients | Cohort | 68 142 | HR 2.0 (95% CI; 1.7-2.3) for 3 or more comorbid conditions vs no comorbidities HR 0.4 (95% CI; 0.3-0.5) for Asian/Pacific Islanders vs Caucasians | 25 |
| Discharge database of University Healthsystem Consortium | Cohort | 1 015 598 | ORs ranging from 1.4 to 1.8 for cancer patients with a comorbidity vs cancer patients without comorbidities | 18 | |
| OR 1.2 and 0.7 for patients with black and asian ethnicity respectively vs white | |||||
| MEGA study | Case-control | 2131/3220 | OR 2.2 (95% CI; 0.3-17.8) for VT in cancer patients with vs without factor V Leiden | 10 |
Cum. inc., cumulative incidence; NA, not applicable; PAR, population attributable risk; PY, person-years; RCT, randomized controlled trial; VT, venous thrombosis.
Cancer patients have a several-fold increased risk of venous thrombosis compared with the general population or patients without cancer, with relative risks (RRs) ranging from 4 to 7 (Table 1).10-13 Frequently cited is the Olmsted County population study. In this study, malignant neoplasm was shown to increase the risk of venous thrombosis fourfold (odds ratio [OR] 4.1; 95% CI: 1.9-8.5).12 However patients were included between 1976 and 1990, which might outdate the findings. In a Dutch population-based case-control study, the MEGA study (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis-study), >3000 consecutive patients with venous thrombosis were included between 1999 and 2004, together with >2100 partner controls.10 The risk of venous thrombosis was increased sevenfold in patients with cancer compared with patients without (OR 6.7; 95% CI: 5.2-8.6). By linkage of 4 United Kingdom databases, Walker and coworkers estimated the RR of venous thrombosis in cancer versus age-matched noncancer controls from the general population to be 4.7 (hazard ratio [HR] 4.7; 95% CI: 4.5-4.9).13 Surprisingly similar results were reported from a Danish population-based cohort of 57,591 incident cancer cases that were followed in time for venous thrombosis, together with 287,476 individuals without cancer from the general population. Noncancer controls were matched for age, gender, and county of residence. After adjustment for comorbid conditions, the risk of venous thrombosis was also 4.7 times higher in cancer patients compared with the noncancer participants (RR 4.7; 95% CI: 4.3-5.1).11 Although these RRs demonstrate a strong association between cancer and venous thrombosis, absolute risks are clinically more meaningful, for example, to communicate a patient’s risk of venous thrombosis or to decide whether a patient needs prophylactic treatment with anticoagulants or not, for which it needs to be balanced with the risk of unwanted side effects (minor or major bleeding) of the anticoagulant treatment. Cohort studies are best suited for this purpose because they provide absolute risks.
The reported absolute risk (cumulative incidence) of venous thrombosis in cancer patients varies widely (1%-8%) depending on patient population, duration of follow-up, calendar period, and the method of detecting and reporting venous thrombotic events (Table 1). The heterogeneity of the studies makes it difficult to compare rates of venous thrombosis between these studies. Some follow-up studies include cancer patients with a diagnosis long before start of follow-up; in others, follow-up is started at the beginning of cancer treatment. When comparing studies and generalizing results to other populations, follow-up should start at the same time, preferably at time of cancer diagnosis. When follow-up starts at a later time, some patients may have died and are therefore missing in the analyses. By linkage of the California Cancer Registry to the California Patient Discharge Data Set, Chew and colleagues followed 235 149 cancer patients from time of cancer diagnosis. Within 2 years, 5032 patients developed a venous thrombotic event (1.6%).14 The cumulative incidence reported in populations of such cancer registries or hospital discharge data are generally lower compared with rates reported in, for example, patients admitted to an inpatient oncology service. This is indeed observed in data from Sallah et al, who reported a cumulative incidence of venous thrombosis of 7.8% in 26 months in cancer patients referred to hematology/ oncology services.15 In the Vienna Cancer and Thrombosis study (CATS) study, a prospective follow-up of 840 cancer patients admitted to the Medical University in Vienna showed that 8% of the cancer patients developed a venous thrombotic event within 1 year after diagnosis or progression of disease.16
A recent meta-analysis by Horsted et al described incidence rates of venous thrombosis in cancer patients, stratified by background risk of venous thrombosis.17 Among cohorts with average-risk patients, defined as cancer patients representative of all patients with cancer, the incidence rate of venous thrombosis was estimated to be 13 per 1000 person-years (95% CI: 7-23). Among cohorts with high-risk patients, defined as cancer patients with high-grade or metastatic disease or treated with therapeutic strategies that increase thromboembolic risk, the overall incidence rate was 68 per 1000 person-years (95% CI: 48-96). In the abovementioned study with linkage of 4 United Kingdom databases, >82 000 cancer patients and >577 000 age-matched control participants were followed in time for venous thrombotic events. The incidence rate of venous thrombosis in all cancers was 13.9 per 1000 person-years (95% CI: 13.4-14.4).13
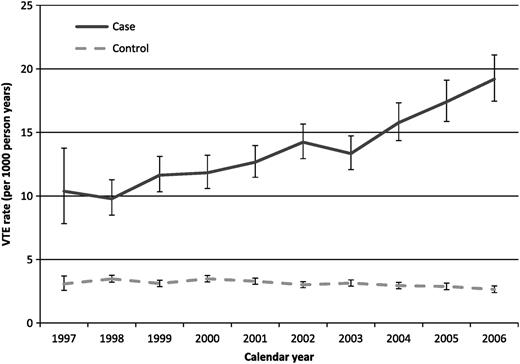
Over the years, the incidence of venous thrombosis in cancer patients has increased (Table 1).18,19 Among patients hospitalized with cancer between 1979 and 1999, the cumulative incidence of venous thrombosis was reported by Stein and coworkers. Data were obtained from the US National Hospital Discharge Survey. The cumulative incidence of venous thrombosis increased from the late 1980s onward (1.5% in 1989), and this trend continued to the late 1990s (3.5% in 1999).19 A similar trend was seen in another study of hospital discharge data. In this study, the cumulative incidence of venous thrombosis was 3.6% in 1995 to 1996 and 4.6% in 2002 to 2003 (28% increase).18 A similar rise in venous thrombosis incidence over time in cancer patients, but not in noncancer controls, is seen in the study with linkage of 4 United Kingdom databases by Walker et al13 (Figure 1). In this study, the rise in VT incidence is reported for different cancer types. Several factors could explain this finding, including a greater awareness of the association between cancer and venous thrombosis and improvements in diagnostic tests. Also, due to improved treatment strategies, patients with cancer currently survive longer, leading to more aged patients undergoing more cancer treatments, which also increases thrombosis risk. For these reasons, the incidence is expected to rise further in the future.
Absolute rates of venous thrombosis (per 1000 person-years) for individual calendar years between 1997 and 2006. Cases are cancer patients and controls are age-matched noncancer controls from the general population. Figure from Walker European Journal of Cancer 2013, with permission from Elsevier.13
Absolute rates of venous thrombosis (per 1000 person-years) for individual calendar years between 1997 and 2006. Cases are cancer patients and controls are age-matched noncancer controls from the general population. Figure from Walker European Journal of Cancer 2013, with permission from Elsevier.13
Risk factors for venous thrombosis in cancer patients
Cancer is a heterogeneous disease, and its different types and stages should be taken into account when determining the risk of venous thrombosis. Also several patient-associated and treatment-associated factors are known to increase the risk of thrombosis.
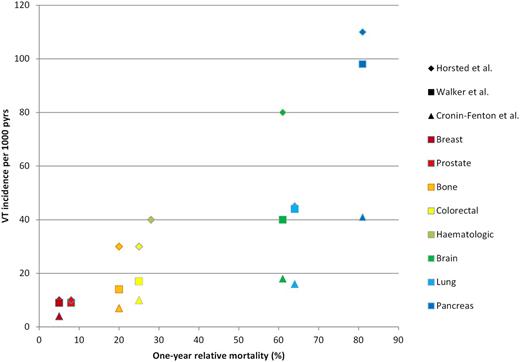
Extensive work has been published on type of malignancy and subsequent risk of venous thrombosis (Table 1). Overall, pancreas, brain, lung, and ovarian cancer are reported to induce highest risks.11,13,17,20 In the literature, high risks are additionally reported for lymphomas, myeloma, and kidney, stomach, and bone cancer.11,14,18,21 Relatively low risks are generally seen in patients with breast or prostate cancer. In their meta-analysis, Horsted and colleagues summarized incidence rates of venous thrombosis for 8 different types of malignancy (Figure 2).17 For the absolute risks presented in this figure, only cohort studies with start of follow-up at time of cancer diagnosis were included. It appears that especially the cancer types that are biologically aggressive, as evidenced by short survival time and early metastatic spread, are correlated with a high incidence of venous thrombosis.22 Figure 3 shows venous thrombosis incidence rates for different types of cancer (according to results of Horsted et al,17 Walker et al, 13 and Cronin-Fenton et al11 ) grouped and plotted against the 1-year relative mortality for each cancer type. One-year relative mortality rates were derived from Eurocare.it.23 Although venous thrombosis incidence per type of cancer varies for the different studies, a clear positive association can be observed with 1-year relative mortality of the cancer type as a measure of biological aggressiveness of the cancer and an associated thrombogenic potential.
Pooled incidence rates (per 1000 person-years) of venous thrombosis per type of cancer. Only studies with start of follow-up at time of cancer diagnosis were included. Numbers in brackets refer to the number of studies that contributed to the pooled estimate. Figure from Horsted Plos Med 2012.17
Pooled incidence rates (per 1000 person-years) of venous thrombosis per type of cancer. Only studies with start of follow-up at time of cancer diagnosis were included. Numbers in brackets refer to the number of studies that contributed to the pooled estimate. Figure from Horsted Plos Med 2012.17
Incidence rates of venous thrombosis (VT) (per 1000 person-years) per type of cancer (according to Horsted et al,17 Walker et al,13 and Cronin-Fenton et al11 ) plotted against the 1-year relative mortality for each cancer type. One-year relative mortality was calculated by (1 – 1-year relative survival) according to Eurocare.it.23 For hematologic cancer, venous thrombosis incidence is exclusively shown for Horsted et al, because Walker et al and Cronin-Fenton et al did not present venous thrombosis incidence rates for hematologic cancer as a combined group.
Incidence rates of venous thrombosis (VT) (per 1000 person-years) per type of cancer (according to Horsted et al,17 Walker et al,13 and Cronin-Fenton et al11 ) plotted against the 1-year relative mortality for each cancer type. One-year relative mortality was calculated by (1 – 1-year relative survival) according to Eurocare.it.23 For hematologic cancer, venous thrombosis incidence is exclusively shown for Horsted et al, because Walker et al and Cronin-Fenton et al did not present venous thrombosis incidence rates for hematologic cancer as a combined group.
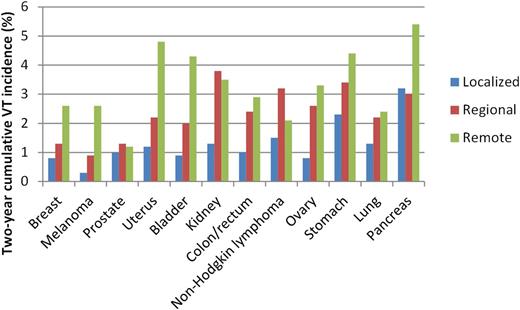
Such an association between aggressiveness of cancer and thrombogenic potential can also be observed when taking stage of cancer into account, which is highly correlated with risk of venous thrombosis (Table 1).10,11,14,17 In the Danish follow-up study mentioned above, where 55 000 cancer patients and >285 000 matched noncancer controls from the general population were followed in time, the risk of venous thrombosis in cancer patients appeared to be strongly dependent on stage of the cancer, with adjusted RRs of 2.9, 2.9, 7.5, and 17.1 among patients with stage I, II, III, and IV disease, respectively.11 Also, in the California Cancer Registry study, increased RRs of venous thromboembolic events in metastatic cancer patients compared with patients with localized disease were reported for 12 different types of cancer (range of HRs, 1.1-21.5).14 In this study, metastatic disease at time of cancer diagnosis was found to be the strongest predictor of subsequent venous thrombosis. Figure 4 shows 2-year cumulative incidence rates of venous thrombosis per type and stage of cancer, according to data from this California Cancer Registry.14 For every type of cancer presented, venous thrombosis incidence increases from localized to regional to remote cancer. Last, in the CATS study, which included 740 patients with newly diagnosed (or progressed after remission) patients with solid tumors, tumor grade (G3+G4 vs G1+G2) was also significantly associated with risk of venous thrombosis (HR 2.0; 95% CI: 1.1-3.5).24 This was after correction for age, gender, tumor histology, types, and stage.
Two-year cumulative incidence (%) of venous thrombosis per type and stage of cancer. Types of cancer were ordered by their respective 1-year mortality rates, according to Eurocare.it.23 Data from Chew et al.14
The incidence of venous thrombosis is clearly highest in the first few months after cancer diagnosis and decreases thereafter (Table 1). In the MEGA study, the risk of venous thrombosis was highest in the first 3 months after cancer diagnosis (OR 53.5; 95% CI: 8.6-334.3), was decreased but still high in the period between 3 and 12 months (OR 14.3; 95% CI: 5.8-35.2), and decreased to almost no elevated risk 10 years after cancer diagnosis.10 In a retrospective analysis of >68 000 colorectal cancer patients from the California Cancer Registry, incidence rates of symptomatic venous thrombosis were calculated.25 The incidence was reported to decrease over time from 5.0/100 person-years in the first 6 months after cancer diagnosis, to 1.4/100 person-years 6 to 12 months after cancer diagnosis, and to 0.6/100 person-years 12 to 24 months after cancer diagnosis. This phenomenon has been shown for all types of cancer in the large follow-up study by linkage of 4 United Kingdom databases.13 This change in risk over time again illustrates why follow-up studies into incidence of venous thrombosis in cancer patients need to start at time of cancer diagnosis. If follow-up is started at a later point in time, the incidence will be lower, and studies cannot be compared directly. There are several possible explanations for a higher risk of venous thrombosis in the first few months after diagnosis compared with the period thereafter. First, several cancer treatment modalities increase the risk of venous thrombosis (see below), inducing a high risk directly after diagnosis and start of treatment. Second, a proportion of treated cancer patients will go into remission, leading to a reduced thrombotic risk thereafter. A third explanation is that over time, a considerable proportion of the cancer patients will succumb to the disease. The occurrence of such a competing event (death) will prevent thrombotic events from being observed.
In addition to type and staging of cancer, cancer treatment modalities also substantially increase the thrombotic potential (Table 1). Surgery, chemotherapy, hormonal therapy, antiangiogenic drugs, immunomodulatory agents, erythropoiesis-stimulating agents (ESAs), blood transfusions, and central venous catheters are all reported to be associated with an increased risk.26,27 Surgery is a well-known risk factor for venous thrombosis, in cancer and noncancer patients. In cancer patients, risk of 90-day postoperative venous thrombosis is reported to be twice as high as in noncancer patients.28 Incidence rates in patients treated with chemotherapy are high, with an annual incidence of 11% to 20%.29 Also, other new systemic cancer treatments and supportive therapies are reported to predispose to venous thrombosis.29 An important caveat, however, in interpreting these risks is that most studies on this topic are observational studies. In observational studies, the decision on (type of) treatment is made by the treating physician, depending on several patient’s characteristics, such as stage of disease and prognosis. Therefore, treated and untreated patients are not directly comparable, and it cannot be discerned whether increased risk of venous thromboembolism is due to the treatment, the cancer, or the patients’ prognosis. This phenomenon is called confounding by indication and plays a role in all observational studies. In randomized clinical trials, exposure (treatment) is assigned in a random fashion, for which reason patients are directly comparable with respect to their thrombotic risk. A direct comparison of different treatment modalities is even more difficult when thrombosis prophylaxis is indicated for specific types of treatment. For example, the risk in patients who underwent surgery cannot be directly compared with the risk in patients treated with chemotherapy, as thromboprophylaxis is common practice after surgery but not during chemotherapy. A disadvantage of clinical trials is the highly selected patient population, limiting the generalizability of the results.
Out of the large amount of literature on this topic, we will present some examples of randomized clinical trials as an illustration of increased risk induced by several types of treatment. Several randomized clinical trials in women with breast cancer have shown a clear link between chemotherapy and/or hormone therapy and venous thrombosis risk.30-33 In a randomized trial in postmenopausal women with node-positive primary operable breast cancer (with positive estrogen and progesterone receptor status), the cumulative incidence of thromboembolic events was assessed for women randomized to 2 years of tamoxifen or to tamoxifen (2 years) plus chemotherapy for 6 months.33 The cumulative incidence in the tamoxifen only group was 2.6% vs 13.6% in the combined treatment group. Similarly, results from a clinical trial in advanced gastro-esophageal cancer patients showed varying rates of venous thrombosis for either 1 of 4 epirubicin/platinum/fluoropyrimidine combination regimens during treatment until 30 days after the last treatment cycle. A higher cumulative incidence of venous thrombosis was observed in patients receiving a cisplatin-containing combination regimen (12.2%) compared with oxaliplatin (6.5%).34 A systematic review of randomized controlled trials demonstrated that cancer patients treated with ESAs in addition to red blood cell transfusions had an increased risk of thromboembolic events over patients not additionally treated with ESAs (RR 1.7).35 These results are supported by a systematic review from Bennett et al.36 In another large meta-analysis of clinical trials, patients with cancer receiving the angiogenesis inhibitor bevacizumab had a somewhat increased risk of venous thrombosis (RR 1.3; 95% CI: 1.1-1.6).37
Apart from cancer-related factors, patient-related factors play a role in the development of thrombosis in cancer patients (Table 1). Several traditional risk factors for thrombosis are additionally present in many cancer patients such as older age, prolonged immobility, prior history of venous thrombosis, and comorbidities. In the California Cancer Registry study in colorectal cancer patients, a significant predictor of venous thrombosis during the first year after diagnosis was the presence of ≥3 comorbid conditions (HR 2.0; 95% CI: 1.7-2.3).25 In a retrospective cohort study using discharge databases of all cancer patients admitted to US academic medical centers, >1 000 000 cancer patients were followed for venous thrombosis.18 Variables associated with venous thrombosis in a clinically significant way were ethnicity and the presence of comorbidities. Such comorbidities included arterial thromboembolism, pulmonary disease, renal disease, infection, and anemia, which all increased the risk of venous thrombosis (ORs 1.5, 1.4, 1.5, 1.8, and 1.4, respectively). Patients with black ethnicity seemed to be at increased risk (OR 1.2; 95% CI: 1.1-1.2), whereas patients with Asian ethnicity had a decreased risk of venous thrombosis compared with whites (OR 0.7; 95% CI: 0.7-0.8). Similarly, in colorectal cancer patients from the abovementioned California Cancer Registry, the risk of venous thrombosis was significantly reduced among Asians/Pacific Islanders (HR 0.4; 95% CI: 0.3-0.4) compared with white patients.25 This is probably explained by an overall lower risk of venous thrombosis in Asians/Pacific Islanders.9 Prothrombotic mutations are additionally reported to influence risk of thrombosis in cancer patients.10,38 For example, the factor V Leiden mutation seems to interact with cancer with respect to venous thrombosis risk. Cancer patients with factor V Leiden were reported to have a twofold increased risk of venous thrombosis compared with noncarriers with cancer (adjusted OR 2.2; 95% CI: 0.3-17.8).10
Clinical presentation
A limited number of studies have looked at differences in the clinical presentation of venous thrombosis between patients with and without cancer. Bilateral DVT seems to be more common among cancer patients than in noncancer patients.39-41 A recent study by Imberti et al showed that rates of symptomatic bilateral lower limb DVT, symptomatic iliocaval thrombosis, and upper limb DVT were higher in cancer patients compared with patients free from cancer (8.5% vs 4.6%, 22.6% vs 14.0%, and 9.9% vs 4.8%, respectively).6 In this study, rates of PE and symptomatic proximal DVT were similar. The relatively high incidence of upper limb DVT in cancer patients is at least partly explained by the frequent use of a central venous catheter.42 Furthermore, cancer is reported to be common in rare forms of thrombosis such as Budd-Chiari syndrome, extrahepatic portal vein obstruction, and mesenteric vein thrombosis.43
Prognosis
In general, cancer patients with venous thrombosis do not fare well. Thrombotic events are reported to be the second leading cause of death in cancer patients.44 Patients with cancer-associated venous thrombosis have higher risks of bleeding complications during anticoagulant treatment and of recurrent venous thrombosis than patients with venous thrombosis but without cancer.4,45,46 In a Norwegian study of 740 patients with a first venous thrombotic event, the 1-year case fatality rates (the proportion of deaths within 1 year after the venous thrombotic event) were fivefold higher in patients with cancer-associated venous thrombosis (63.4%; 95% CI: 54.5-71.8) than in venous thrombosis patients without cancer (12.6%; 95% CI: 10.1-15.5).7 In the RIETE registry, a large prospective cohort of >35 000 VT patients, 3-month mortality was much higher in the patients with cancer-related venous thrombosis compared with venous thrombosis patients without cancer (26% vs 4%, respectively).4
Furthermore, cancer patients who develop a venous thrombotic event have a lower survival rate than cancer patients without venous thrombosis.14,47-50 In a large Danish population-based study, patients diagnosed with cancer at the time of venous thrombosis were matched to control cancer patients without venous thrombosis, based on age, gender, type of cancer, and year of diagnosis.50 The 1-year survival rate for the group with cancer and venous thrombosis was 12% compared with 36% in the control group. Chew and colleagues investigated the survival of >235 000 cancer patients and compared these survival rates between cancer patients with and without a subsequent diagnosis of venous thrombosis.14 In a multivariate analysis with adjustment for age, race, and stage of cancer, a diagnosis of venous thrombosis was a significant predictor of decreased survival within 1 year for all cancer types (HRs ranging from 1.6 to 4.2). We studied mortality rates in participants of the Tromsø study, a large Norwegian follow-up study in participants free of cancer and venous thrombosis at baseline in 1994 to 1995.3 In total, 25 983 subjects were followed until September 1, 2007, of whom1751 subjects developed cancer and 417 developed venous thrombosis (109 of which were cancer related). By means of a time-dependent analysis, mortality rates and HRs for death were estimated for disease-free subjects, subjects with cancer only, subjects with venous thrombosis only, and subjects with cancer-related venous thrombosis (Table 2). Subjects with cancer-related venous thrombosis had a 30-fold increased risk of death during follow-up compared with disease-free subjects (HR 31.2; 95% CI: 24.6-39.6), whereas subjects with cancer only or venous thrombosis only had a sevenfold and threefold increased risk, respectively. An explanation for the difference in mortality rates could be the more aggressive course of the malignancies associated with high thrombosis risk (Figure 3). It is unknown to what extent the high mortality rates in patients with cancer and venous thrombosis can be attributed to the thrombotic events themselves. In a study in 4466 cancer patients in the United States starting with chemotherapy and followed for a median of 75 days, thrombosis (including both venous and arterial events) was the second leading cause of death (n = 13; 9%) after cancer progression (n = 100; 71%).44 In this study, causes of death were assigned by the treating physicians, mainly based on clinical data, rather than autopsies. Among patients from a large database comprised of Multiple-Cause Mortality Files from 1979 to 1998 in whom PE was reported on the death certificates, 23% were reported to have cancer.51 Causes of death according to the treating physician or death certificate may not be that reliable, and autopsy studies should be used to answer this question. In 2 autopsy studies from Sweden and the United States, the incidence of PE in cancer patients was 26% and 17%, respectively, of which 8% and 14% were fatal pulmonary emboli.52
Crude mortality rates and age- and gender-adjusted HRs of death in participants without cancer and without venous thrombosis, with venous thrombosis only, with cancer only, and with cancer-related venous thrombosis (The Tromsø study 1994-2007)
| Exposure . | PY . | Deaths (n) . | MR per 100 PY (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|
| None | 277 713 | 1750 | 0.63 (0.60-0.66) | 1.0 (reference) |
| VT only | 1317 | 67 | 5.1 (4.0-6.4) | 2.6 (2.0-3.3) |
| Cancer only | 5650 | 721 | 12.7 (11.9-13.7) | 7.4 (6.8-8.2) |
| Cancer-related VT | 131 | 72 | 55.0 (43.6-69.3) | 31.2 (24.6-39.6) |
| Exposure . | PY . | Deaths (n) . | MR per 100 PY (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|
| None | 277 713 | 1750 | 0.63 (0.60-0.66) | 1.0 (reference) |
| VT only | 1317 | 67 | 5.1 (4.0-6.4) | 2.6 (2.0-3.3) |
| Cancer only | 5650 | 721 | 12.7 (11.9-13.7) | 7.4 (6.8-8.2) |
| Cancer-related VT | 131 | 72 | 55.0 (43.6-69.3) | 31.2 (24.6-39.6) |
HRs were calculated by means of a time-dependent Cox regression analysis. MR, mortality rate; PY, person-years; VT, venous thrombosis.
Thromboprophylaxis
It is hypothesized that anticoagulant treatment for the prevention of venous thrombotic events in cancer patients might improve prognosis and quality of life. However, such treatment comes with a disadvantage of an increased risk of bleeding, which is especially pronounced in cancer patients.46,53,54 In a prospective follow-up of 842 DVT patients, Prandoni et al investigated bleeding rates during anticoagulant treatment. The 12-month cumulative incidence of major bleeding was about twofold higher in patients with active cancer (12.4%; 95% CI: 6.5%-18.2%) than in patients without cancer (4.9%; 95% CI: 2.5%-7.4%).46 Several randomized clinical trials have investigated the effects of thromboprophylaxis in ambulatory cancer patients receiving chemotherapy. A recent Cochrane review summarized results of 9 of those trials.55 Thromboprophylaxis was reported to significantly reduce the incidence of symptomatic venous thrombosis (RR 0.62; 95% CI: 0.41-0.93). However, this treatment was also associated with an increase in bleeding events. The number needed to treat to prevent 1 venous thrombotic event was 60. Thromboprophylaxis should therefore be targeted only at cancer patients with a high risk of venous thrombosis, which outweighs the risk of bleeding events. Several biomarkers have been associated with risk of venous thrombosis in cancer patients, such as P-selectin, d-dimer, tissue factor–bearing microparticles (TFMP), prechemotherapy hemoglobin, platelet and leukocyte counts, factor VIII, and C-reactive protein.16,56-63 A recent clinical trial randomized advanced cancer patients with higher levels (>3.5 × 104 microparticles/µL) of circulating TFMP to either enoxaparin for 2 months (n = 23) or observation without any treatment (n = 11).64 Advanced cancer patients with lower levels of TFMP were followed without treatment (n = 32). Patients with higher TFMP levels, not randomized to enoxaparin, had a significantly higher 2-month cumulative incidence of venous thrombosis (27%) compared with patients with lower TFMP levels (7%). Patients with high TFMP levels randomized to enoxaparin had the lowest cumulative incidence of venous thrombosis (6%). Median survival was 17.8 months in patients treated with enoxaparin compared with 11.8 months in untreated patients with higher levels of TFMP.
Although this clinical trial using risk stratification based on 1 biomarker shows promising results, prediction models incorporating several risk factors, instead of 1, are probably more useful for guiding decisions on prophylaxis in individual patients. Such a risk assessment model has been developed by Khorana et al.59 In a randomly selected development cohort of 2701 cancer patients initiating a new chemotherapy regimen, baseline clinical and laboratory risk factors for venous thrombosis were included in a risk model, which was validated in an independent cohort of 1365 cancer patients from the same population. Patients were followed for symptomatic venous thromboembolic events for a median of 73 days. Five predictive variables present before initiation of chemotherapy were identified in the final multivariate analysis and used for a risk score model: primary site of cancer, platelet count ≥350 000/μL, hemoglobin >10 g/dL, and/or use of red cell growth factors, leukocyte count >11 000/μL, and body mass index ≥35 kg/m2 (Table 3). Rates of venous thrombosis in the development and validation cohort were 0.8% and 0.3% in low-risk (score = 0), 1.8% and 2% in intermediate-risk (score = 1-2), and 7.1% and 6.7% in high-risk patients (score ≥ 3), respectively. Ay and colleagues applied this risk model to their prospective observational cohort study of patients with newly diagnosed cancer or with progression of disease after complete or partial remission who had not recently received chemotherapy, surgery, and/or radiotherapy (CATS study).65 Additionally, they expanded the model by adding 2 predictive biomarkers, ie, soluble P-selectin (≥53.1 ng/mL) and d-dimer levels (≥1.44 μg/mL), and they added additional types of cancer to the high- and very-high-risk groups. In the expanded risk model, the cumulative probabilities of venous thrombosis after 6 months of follow-up were 35% in patients with a score ≥5, 10.3% in patients with a score of 3, and 1.0% in patients with a score of 0. The disadvantage of this expanded risk model is that additional laboratory tests have to be performed because d-dimer and P-selectin levels are not routinely measured in the clinic. Intervention trials based on risk assessment models are necessary to demonstrate the effectiveness and safety of prophylactic anticoagulant treatment in high-risk patients. In an ongoing study, the use of thromboprophylaxis in patients deemed high risk, based on the original prediction model by Khorana et al, is currently being tested (www.clinicaltrials.gov No. NCT00876915).
Predictive model for chemotherapy-associated venous thrombosis
| Patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Prechemotherapy platelet count ≥350 × 109/L | 1 |
| Hemoglobin level < 100 g/L or use of red cell growth factors | 1 |
| Prechemotherapy leukocyte count >11 × 109/L | 1 |
| Body mass index ≥ 35 kg/m2 | 1 |
| Patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Prechemotherapy platelet count ≥350 × 109/L | 1 |
| Hemoglobin level < 100 g/L or use of red cell growth factors | 1 |
| Prechemotherapy leukocyte count >11 × 109/L | 1 |
| Body mass index ≥ 35 kg/m2 | 1 |
From Khorana et al.59
Recurrent venous thrombosis and cancer
The overall risk of recurrent venous thrombosis in patients who suffered once from venous thrombosis is high, with a 5- to 10-year cumulative incidence ranging from 25% to 30%.66-68 Cancer patients are at an approximately two- to threefold increased risk of recurrent venous thrombosis compared with noncancer patients.46,67-69 Prandoni and coworkers followed 355 consecutive patients with a first episode of DVT for 8 years and found a twofold risk of recurrent venous thrombosis in cancer patients compared with noncancer patients (HR 1.7; 95% CI: 1.3-2.3).68 The same group of investigators found a 12-month cumulative incidence of recurrent venous thrombosis of 20.7% in cancer patients on conventional anticoagulant treatment vs 6.8% in patients without cancer on anticoagulant treatment in a prospective cohort study including 842 DVT patients.46 Recurrence appeared to be related to extent of disease, classified according to the tumor node metastasis classification, with highest recurrence rates in patients with extensive vs moderately or less extensive cancer. This again reflects the apparent relation between aggressiveness of cancer and thrombogenic potential. In the RIETE study, patients with symptomatic, acute venous thrombosis were enrolled, and 3-month outcomes of the participants were studied. Of 18 883 participants, 3805 had been diagnosed with active cancer. A RR for recurrent PE of 2.0 and for recurrent DVT of 2.4 was found for patients with a cancer diagnosis >3 months before their first venous thrombosis.69 Not much is known about the risk of recurrent venous thrombosis for different types of cancer, and results from previous studies are contradictory.46,69 A clinical prediction rule (Ottawa prognostic score) has been developed for recurrent venous thrombosis during the first 6 months of anticoagulant treatment in a retrospective cohort study of 543 patients with a cancer-associated venous thrombotic event.70 The final model included 4 predictors (gender, primary tumor site, stage, and number of prior venous thrombotic events) leading to a score sum that ranged between −3 and +3 points. Patients with a score ≤0 had a low risk of recurrence (4%), whereas patients with a score ≥1 had a relatively high recurrence risk (16%). The prediction rule was validated by the investigators in an independent set of patients from 2 randomized clinical trials, and results appeared to be consistent. Another group of investigators from the Netherlands assessed the reproducibility of the Ottawa score in an independent sample of 419 patients with cancer-associated venous thrombosis.71 Their results were similar to those reported by Louzada and coworkers in their validation sample. Recently the Ottawa score was additionally validated in an independent patient population in a tertiary hospital in Korea.72 In 546 patients with cancer-associated venous thrombosis, the model was less discriminatory compared with the derivation study. Of patients in the low-risk group (score ≤ 0), 13.2% were identified with recurrent venous thrombosis, whereas 22.4% of patients in the high-risk group (score ≥ 1) were identified with a recurrence. Thrombosis risk and cancer predominance are known to be different in the Asian population, which may be an explanation for the different findings. Furthermore, differences in study design, such as different durations of follow-up or definition of recurrences, may explain these findings.
Screening
Acute venous thrombosis can be the first manifestation of an occult cancer. Rates of occult cancer detection at the time or shortly after diagnosis of venous thrombosis vary in the literature, depending on patient population, duration of follow-up, and detection methods. Although some articles published in the 1980s contradict each other as to whether there is an association between venous thrombosis and an increased risk of subsequent cancer diagnosis,73-75 recent articles show a clear association between the two. In a nationwide, retrospective cohort study in Scotland, almost 60,000 patients with DVT or PE diagnosed between 1982 and 2000 were followed for the occurrence of cancer until the end of 2000.76 The ratio of the observed cases of cancer and the number of cases expected based on national cancer incidence rates was calculated, which gives a standardized incidence ratio (SIR). For all malignancies combined, there was an excess risk of being diagnosed with cancer in venous thrombosis patients, which remained up to 2 years after diagnosis of venous thrombosis event. Especially in the first 1 to 6 months after diagnosis of venous thrombosis, the risk was high (SIR 4.2; 95% CI: 3.9-4.5). Two other follow-up studies, quite alike in design, showed similar results with respect to risks and types of cancer (liver, pancreas, ovary, brain, and lymphoma), for which the association was most pronounced.77,78 In a recent systematic review by Carrier and colleagues, data from 34 studies that reported prevalence of undiagnosed cancer at the time of an acute first thromboembolic event were combined.79 In 4.1% (95% CI: 3.6%-4.6%) of the included patients, a previously undiagnosed cancer was detected within a month after the venous thrombotic event. Within a year after the event, 6.3% (95% CI: 5.6%-6.9%) of the patients were diagnosed with cancer.
Patients with an idiopathic venous thrombosis have a higher risk of detection of an occult cancer than patients with a venous thrombotic event secondary to a provoking risk factor.79,80 In the abovementioned study by Carrier et al, the period prevalence of previously undiagnosed cancer between baseline (venous thrombotic event) and 12 months was 10.0% (95% CI: 8.6%-11.3%) for patients with unprovoked venous thrombosis vs 2.6% (95% CI: 1.6%-3.6%) for patients with a secondary event. This raises the question of whether only patients with an idiopathic venous thrombosis should be screened for occult cancer. Van Doormaal and colleagues prospectively followed 630 idiopathic venous thrombosis patients who underwent either baseline cancer screening (consisting of history, physical examination, basic laboratory tests, and chest X-ray) or extensive cancer screening (consisting of additional abdominal and chest computed tomography scans and mammography), based on the center in which patients were treated.81 After baseline screening, 7 of 288 patients (2.4%) were diagnosed with cancer vs 12 of 342 patients (3.5%) after extensive screening methods. Survival did not differ between the groups, which led the authors to conclude to not support extensive routine screening for cancer in patients with a first episode of idiopathic venous thrombosis. In 1 randomized clinical trial by Piccioli and colleagues,82 acute idiopathic venous thrombosis patients were randomized to either an extensive screening for occult cancer or to no further testing. Unfortunately, the trial was terminated prematurely due to a lower than anticipated number of participating centers and an increasing tendency among physicians to perform screenings tests for occult cancer in control patients. Extensive screening was found to be able to detect hidden malignancies and to lead to identification of malignancies at an earlier stage. However, due to the limited sample size, effects on prognosis of patients remained unclear. Cancer-related mortality during the 2-year follow-up period did not significantly differ between both groups (absolute difference 1.9%; 95% CI: −5.5% to 10.9%). The effect of extensive screening in idiopathic venous thrombosis patients on prognosis remains elusive.83,84 Further studies are needed to investigate whether screening procedures are cost-effective and affect cancer-related mortality.
Superficial venous thrombosis and cancer
Superficial vein thrombosis (SVT), or superficial thrombophlebitis, is a common condition; the incidence in general has thus far not been properly assessed, possibly because in the past, SVT was considered a benign, self-limiting, disease. However, it is thought to occur at least as often as DVT. Interest in the disease was renewed when more and more studies in the last decade described an association between SVT and DVT.12,85,86 Many conditions have been reported to predispose to SVT, mostly also well-known risk factors for DVT. For this reason, it would be reasonable to suspect an association between cancer and SVT.87-90 The incidence of SVT in cancer patients has not been studied. Whether SVT should be seen as a marker of occult cancer is also controversial. In a substudy of the Calisto trial, a trial in which ∼3000 SVT patients with isolated SVT were randomized to either fondaparinux or placebo, Prandoni and coworkers compared 737 SVT patients with 1438 control patients with regard to cancer diagnoses during an average of 26 months of follow-up.91 They concluded that occurrence of SVT in the legs does not represent a risk factor for subsequent malignancies. The same conclusion was drawn in a small study performed in the Netherlands.92 However, Sorensen et al did find a relation between a diagnosis of SVT and a subsequent cancer diagnosis in the Danish population.93 The occurrence of cancer in 7663 SVT patients was compared with the expected number of cancer diagnoses based on national incidence rates, and a SIR of 2.5 (95% CI: 2.1-2.9) for the first year of follow-up was reported. A possible explanation for the difference in findings is that in the study by Sorensen unrecognized concomitant DVT was possibly present, which increased the risk of a cancer diagnosis. Prandoni and colleagues excluded cases with a concomitant venous thrombotic event confirmed by ultrasonography. Future epidemiologic studies are needed to study the strength of the relationship between SVT and cancer and the incidence of SVT in cancer patients.
Concluding remarks
Despite the fact that the strong association between cancer and venous thrombosis has been known for >150 years, cancer-associated thrombosis is still a topic of extensive (epidemiologic) research from which there is much to gain for patients. Future studies need to be targeted at development and validation of prediction models to categorize cancer patients into high or low risk of venous thrombosis. Randomized trials should study the benefit of thromboprophylaxis in patients deemed at high risk based on these models. Furthermore, studies are needed to investigate whether cancer screening procedures in idiopathic venous thrombosis patients are cost-effective and affect cancer-related mortality.
Acknowledgments
This work was supported by Netherlands Heart Foundation grant NHS2010B167.
Authorship
Contribution: J.F.T. wrote the initial version of the manuscript and integrated the input from coauthors in the final version; S.C.C. defined the general scope of the manuscript and revised successive manuscript versions; and S.K.B. and H.H.V. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S.C. Cannegieter, Department of Clinical Epidemiology C7-P, PO Box 9600, 2300 RC, Leiden, The Netherlands; e-mail: S.C.Cannegieter@lumc.nl.