Key Points
Patients with mastocytosis feature increased NT serum levels and elevated expression of modified NT receptors on skin and gut MCs.
NTs might contribute to mastocytosis via increased migration of MC progenitors, MC differentiation, proliferation, and/or survival.
Abstract
Mastocytosis is a rare heterogeneous disease characterized by increase of mast cells (MCs) in different organs. Neurotrophins (NTs) have been shown to promote differentiation and survival of MCs, which in turn represent a major source of NTs. Thus, a contribution of NTs to mastocytosis seems highly conceivable but has not yet been investigated. We could demonstrate expression of high-affinity NT receptors tropomyosin-related kinase A (TrkA) for nerve growth factor (NGF)-β, TrkB for brain-derived neurotrophic factor, and NT-4 and TrkC for NT-3 on skin MCs; and of TrkA and TrkC on intestinal MCs of patients with mastocytosis. Moreover, increased expression of NGF-β; NT-3; TrkA, TrkB, and TrkC; and isoforms truncated TrkB-T1 and truncated TrkC were observed on skin MCs. Patients with mastocytosis featured elevated serum levels of NGF, NT-3, and NT-4. Levels of NGF-β and NT-4 correlated with tryptase levels, suggesting a link between MC load and blood levels of NGF and NT-4. Migration of CD117+ progenitor cells from the blood was enhanced toward NGF-β gradient in both mastocytosis and controls. Together with enhanced NT levels, the elevated expression of modified Trk receptors on skin and gut MCs might contribute to the pathophysiology of mastocytosis in autocrine and paracrine loops.
Introduction
Mastocytosis is a rare, heterogeneous disease characterized by an abnormal increase of clonal mast cells (MCs) in 1 or more organs.1 From a clinical standpoint, cutaneous, gastrointestinal, cardiovascular, and neuropsychiatric symptoms and an increased risk for anaphylaxis result from the infiltration of clonal MCs, increased body burden of these MCs, and the release of MC mediators.
Cutaneous mastocytosis (CM) is limited to the skin. Systemic mastocytosis (SM) affects other organs in addition to the skin, mostly the bone marrow (BM) or gastrointestinal tract. Most of adult patients experience indolent SM (ISM) without extracutaneous organ dysfunction. ISM is accompanied by cutaneous involvement but also manifests as isolated BM mastocytosis (IBMM) without skin involvement, with a favorable prognosis. Approximately 40% of patients with SM featured an associated clonal hematologic non-MC lineage disease (SM-AHNMD), 12% an aggressive SM (ASM), and 1% MC leukemia (MCL) in a recent study.2,3 In SM-AHNMD, the prognosis is determined by the associated hematologic disease. ASM and MCL are characterized by pronounced organ infiltration with organ dysfunction and cytopenia (C-findings) with a poor prognosis. Smoldering SM (SSM) is a variant of ISM characterized by the presence of ≥2 B-findings (organomegaly without functional impairment, dysmyelopoiesis, serum tryptase level >200 ng/mL, and/or pronounced BM >30%) without C-findings.1,3
Human MCs are derived from CD34+ BM progenitor cells under the influence of growth factors, particularly stem cell factor (SCF), the ligand for KIT (CD117), which regulate the development of MCs.4 Progenitor cells enter the circulation from BM and migrate into the peripheral tissues where they differentiate into their final phenotype in response to SCF and, most likely, additional local factors. Mechanisms directing these progenitors to migrate to and develop in the skin of healthy individuals as well as in patients with CM, or once mature, to release their mediators, are only partly understood.
Gain-of-function mutations in c-kit, the gene for KIT, play a major role in the pathophysiology of mastocytosis,1,5 probably leading to the development of greater numbers of MCs and increasing their survival and susceptibility to become activated. However, KIT mutations are not detected in all patients with mastocytosis and have also been found in other neoplastic diseases.1,5 Moreover, a clear phenotype-genotype correlation could not be shown yet. Furthermore, mutations in the tumor suppressor gene TET25 as well as IL13- and IL4R- polymorphisms and an overexpression of the antiapoptotic proteins Bcl-2/xL6 have been observed in some patients with mastocytosis.
Therapy for mastocytosis is mostly symptomatic and includes avoidance of trigger factors; therapy for MC mediator release; phototherapy; and therapy for osteoporosis, if present. Cytoreductive therapy is applied in advanced forms of SM, especially ASM or MCL. SM-AHNMD, the AHNMD component of the disease, is treated as in patients without SM. To date, no curative treatment of advanced SM exists.3,7
Neurotrophins (NTs) are growth factors that were initially discovered in the nervous system and play a pivotal role in the development, maintenance, and regeneration of nerve fibers. The NTs include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT-3, NT-4, and NT-5. NT precursors, pro-NGF/BDNF are high-affinity ligands for the pan-NT receptor p75 (p75NTR), which together with sortilin, promotes apoptosis through the regulation of the rate of p75 cleavage by sortilin, a prerequisite for pro-NT–initiated cell death.8 Conversely, all mature NTs bind with low affinity to the p75NTR and with high affinity and specificity to the tropomyosin-related kinase family receptors, TrkA for NGF, TrkB for BDNF, and NT-4, and TrkC for NT-3.8 Each full-length (FL) Trk receptor contains an extracellular, transmembrane, and intracellular domain. Binding of NTs to the extracellular domains of Trk receptors leads to dimerization of the receptors, and autophosphorylation of tyrosines in the intracellular domain with activation of downstream signaling pathways including mitogen-activated protein kinase, PI3K/Akt, phospholipase C-γ, and protein kinase C that favor cellular differentiation, proliferation, and survival.8 Alternative splicing during gene transcription generates receptor isoforms with different functions. Besides the FL receptor, TrkA has 2 variants: TrkA I and II, with insertions in the extracellular domain, whereas truncated receptors lacking the intracellular kinase domain have been only discovered for TrkB and TrkC.9-12 Although functions of these receptor isoforms are still not clearly understood, accumulating evidence suggests a contribution to cell growth and migration.13 NTs have also been demonstrated to act as signal transduction molecules between immune cells, structural cells, and neuronal cells in allergic diseases.14-16
NT-expressing nerves are in direct anatomic contact with MCs, and MC-driven skin inflammation has been shown to be impaired in the absence of sensory nerves.17 NTs have been demonstrated to promote chemotaxis, maturation, and survival of MCs, which, on the other hand, produce several NTs.9,14,15,18-26
The bidirectional interaction of NTs and MCs suggests a contribution of NTs to a disease such as mastocytosis. However, the role of NTs in mastocytosis has not been investigated sufficiently so far.
Therefore, our study aimed to further investigate the role of NTs and their receptors in mastocytosis.
Materials and methods
Study population
Patients with mastocytosis (n = 74; 22 men and 52 women; mean age, 50.0 ± 14.4 years; age range, 12-77 years) and healthy control participants (n = 50; 19 men and 31 women; mean age, 48.8 ± 21.6 years; age range, 20-91 years) from the Department of Dermatology and Allergy and the Department of Plastic Surgery, University of Bonn, Germany, were included in the study after giving their informed consent. Recruitment criteria were a clinically and histologically confirmed mastocytosis in the skin (MIS), CM, and/or SM. The group of patients with mastocytosis consisted of 14 patients with CM; 2 patients with ASM; 3 patients with SM-AHNMD; and 40 patients with ISM, whereof 33 had skin involvement, 4 had IBMM, and 3 had SSM according to the World Health Organization criteria.27 The World Health Organization criteria for SM were not assessed in 15 additional patients with MIS because of low serum tryptase levels, absence of systemic symptoms, and/or refusal of the patient to undergo BM biopsy.1 Clinical characteristics of the study population are outlined in supplemental Table 1. A part of the study population has already been described previously.28 The protocol was approved by the local ethics committee, and our current study was conducted in accordance with the Declaration of Helsinki.
Tissue processing
Tissues were fixed in 4% formaline, decalcified (bone marrow biopsies), paraffine embedded and further processed for tissue staining and molecular genetic analysis. Details on tissue processing and identification of KIT mutations are described in supplemental Methods, part 1.
Reagents and serum parameter analysis
Reagents and analysis of serum parameters are described in supplemental Methods, part 2.
Isolation and culture of human SMCs from skin biopsies
Isolation of CD117+ cells from the peripheral blood
Peripheral blood mononuclear cells were isolated from patients with mastocytosis and from buffy coats of healthy blood donors from the blood bank of the University of Bonn by the Lymphoprep gradient technique, as described in the manufacturer's protocol (Axis Shield, Oslo, Norway). CD117+ cells were isolated from peripheral blood mononuclear cells by immunomagnetic selection with mAb against human CD117 (c-kit) (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's instructions, with some minor variations. The purity of CD117+ cells was >90% as measured by flow cytometric staining.
Flow cytometry
RNA isolation and real-time polymerase chain reaction (PCR)
Messenger RNA (mRNA) from cultured SMCs was isolated with the NucleoSpin RNA II Kit (Macherey-Nagel, Düren, Germany), including digestion of genomic DNA, and was subjected to complementary DNA (cDNA) synthesis with TaqMan reverse transcription reagents with random hexamers according to the manufacturer's instructions (Applied Biosystems, Darmstadt, Germany). The prepared cDNA was amplified using TaqMan assay Master Mix (Applied Biosystems) according to the recommendations of the manufacturer in an ABI Prism 7300 Sequence Detection System (Applied Biosystems). Expression of TrkA (Hs01021018_m1), TrkB (Hs01093103_m1), TrkC (Hs00983870_m1), NGF-β (Hs00171458_m1), NT-3 (Hs00267375_s1), and NT-4/5 (Hs01596132_m1) in relationship to an 18s ribosomal RNA endogenous control (4310893E) was evaluated using TaqMan Assays (Applied Biosystems). Relative quantification and calculation of the range of confidence was performed using the comparative ΔΔCT methods.32 All analyses were conducted in duplicates.
Detection of transcript variants of Trk receptors with PCR
To analyze the expression of transcript variants of TrkA, TrkB, and TrkC on cultured SMCs from patients with mastocytosis and control participants, primers were synthesized (Microsynth, Balgach, Switzerland) (supplemental Table 1) and PCRs performed as described previously.9,12,33 The amount of human template cDNA in different PCRs was determined with a parallel PCR for a 114bp fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene with following primers, GAPDH_forward: CCA CAT CGC TCA GAC ACC AT and GAPDH_reverse: GGC AAC AAT ATC CAC TTT ACC AGA GT.11 In each PCR, human brain cDNA and distilled water were used as positive and negative controls, respectively.
Immunohistochemistry staining
Immunohistochemistry was performed as described previously,34 using serial paraffin-embedded sections (4 µm) with the help of mAbs to TrKA, TrKB, and TrKC from R&D Systems and toluidine blue. Appropriate isotype-matched controls were included. TrkA, TrkB, TrkC, and toluidine blue–positive cells were counted in 600 consecutive fields per section (300 upper dermis, 300 lower dermis; magnification ×200; field diameter, 0.05 mm2; total area, 30 mm2) under the microscope (BH-2 microscope and DF70 camera; Olympus Europe GmbH, Hamburg, Germany), and the mean of Trk+ or toluidine blue+ cells/mm2 was calculated.
Immunofluorescence staining
Immunofluorescence staining was performed on 4-µm skin and gut sections of paraformaldehyde-fixed and paraffin-embedded tissue as described elsewhere.35 Sections were treated with mAb against tryptase (Dako, Hamburg, Germany) at 4°C overnight. TrkA, TrkB, and TrkC were analyzed with polyclonal rabbit antibodies or with an appropriate isotype-matched control. Antibody binding was detected with appropriate anti-mouse and anti-rabbit Cy2- and Cy3-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were analyzed with a fluorescence microscope (Leica Microsystems, Wetzlar, Germany) using Diskus and ImageJ software packages (http://rsb.info.nih.gov/ij/) for documentation and analysis.
Enzyme-linked immunosorbent assays (ELISAs)
Serum samples were collected in separator tubes, centrifuged and further processed for ELISAs as described in supplemental Methods, part 4.
Migration assays
Cell migration was performed in 24-well transwell cell culture chambers (Costar, Corning, NY) in accordance with published protocols,36 with some minor variations (supplemental Methods, part 5).
Statistical analysis
Differences between the groups were assessed using the χ2 test for qualitative parameters, the Mann-Whitney U test for NTs, tryptase, and IgE ELISA data, and the Spearman ρ test for correlations. Real-time PCR data were analyzed with the t test. Log-transformed migration data were analyzed using repeated-measurement analysis of variance with Bonferroni-corrected pairwise comparison of conditions for both groups and a paired-sampled t test, when looking at separate groups (SPSS 21.0; SPSS Inc., Chicago, IL). Results are given as mean ± standard deviation or standard error of the mean (SEM), respectively. Any P values are 2 sided, and P < .05 is considered statistically significant. Except for the repeated-measurement analysis of variance, no correction for multiple testing was made for other descriptive tests and P values.
Results
Increased serum levels of NGF-β, NT-3, and NT-4 in patients with mastocytosis
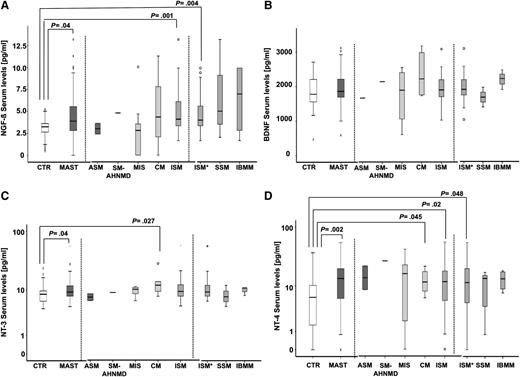
We observed significantly increased serum levels of NGF-β (P = .04), NT-3 (P = .04), and NT-4 (P = .002) in patients with mastocytosis compared with control participants (Figure 1). NGF-β levels were highest in patients with IBMM but did only reach statistical significance in patients with ISM with skin involvement (P = .004) and in all patients with ISM (P = .001). Conversely, patients with pure CM had the highest levels of BDNF, NT-3 (P = .027), and, similar to MIS, of NT-4 (P = .045). Also, patients with ISM showed significantly higher levels of NT-4 (P = .02) compared with control participants. In the analysis of the separate subgroups of ISM, a narrow significance was reached (P = .048) only in patients with ISM and skin involvement, but not in patients with SSM or IBMM. There were no significant differences between BDNF levels in patients with mastocytosis compared with control participants or between NT levels of the different mastocytosis variants.
Increased serum levels of NGF-β, NT-3, and NT-4 in patients with mastocytosis. NGF-β (A), BDNF (B), NT-3 (C), and NT-4 (D) serum levels of healthy control participants (CTR; n = 50 for NGF-β, NT-4; n = 33 for BDNF; n = 40 for NT-3) and patients with mastocytosis (MAST; n = 49 for NGF-β, NT-4; n = 33 for BDNF; n = 39 for NT-3). ASM (n = 2 for NGF-β; NT-3, NT-4; n = 1 for BDNF), SM-AHNMD (n = 1 for all NTs), MIS (n = 11 for NGF-β, NT-4; n = 6 for BDNF; n = 5 for NT-3); CM (n = 8 for NGF-β, NT-4; n = 6 for BDNF; n = 6 for NT-3); ISM (n = 27 for NGF-β, NT-4; n = 25 for NT-3, n = 19 for BDNF), ISM with skin involvement (ISM*) (n = 20 for NGF-β, NT-4; n = 19 for NT-3, n = 13 for BDNF), SSM (n = 3 for all NT), and IBMM (n = 4 for NGF-β, NT-4; n = 3 for BDNF, NT-3).
Increased serum levels of NGF-β, NT-3, and NT-4 in patients with mastocytosis. NGF-β (A), BDNF (B), NT-3 (C), and NT-4 (D) serum levels of healthy control participants (CTR; n = 50 for NGF-β, NT-4; n = 33 for BDNF; n = 40 for NT-3) and patients with mastocytosis (MAST; n = 49 for NGF-β, NT-4; n = 33 for BDNF; n = 39 for NT-3). ASM (n = 2 for NGF-β; NT-3, NT-4; n = 1 for BDNF), SM-AHNMD (n = 1 for all NTs), MIS (n = 11 for NGF-β, NT-4; n = 6 for BDNF; n = 5 for NT-3); CM (n = 8 for NGF-β, NT-4; n = 6 for BDNF; n = 6 for NT-3); ISM (n = 27 for NGF-β, NT-4; n = 25 for NT-3, n = 19 for BDNF), ISM with skin involvement (ISM*) (n = 20 for NGF-β, NT-4; n = 19 for NT-3, n = 13 for BDNF), SSM (n = 3 for all NT), and IBMM (n = 4 for NGF-β, NT-4; n = 3 for BDNF, NT-3).
NT levels correlated among each other. NGF-β correlated positively with NT-3 (rs=0.48; P = 7.8*10−6) and BDNF (rs=0.35; P = .004). Conversely, NT-4 correlated negatively with NGF-β (rs=−0.21; P = .004) and NT-3 (rs=−0.25; P = .02). It is interesting to note that NGF-β (rs=0.35; P = 4.5*10−4) and NT-4 (rs=0.31; P = .002) serum levels correlated with serum tryptase levels, implying a direct link between MC load37 and NGF-β and NT-4 blood levels in patients with mastocytosis. We did not observe significant differences in NT levels between patients with mastocytosis carrying an activating KIT mutation compared with those without such a mutation, analyzed in a subgroup of patients (n = 24; data not shown).
mRNA expression of NGF-β, NT-3, TrkB, and TrkC is increased in the SMCs of patients with mastocytosis
Patients with mastocytosis exhibited a higher mRNA expression of NGF-β (P = .05), NT-3 (P = .02), NT-4/5 (not significant), TrkB (P = .04), and TrkC (P = .03), but only slightly increased TrkA (not significant) mRNA expression in cultured SMCs (Figure 2) compared with control participants.
Elevated NGF, NT-3, TrkB, and TrkC mRNA expression on SMCs from patients with mastocytosis. Relative mRNA expression of NGF-β (A), NT-3 (B), NT-4/5 (C), TrkA (D), TrkB (E), and TrkC (F) on cultured SMCs isolated from healthy skin (CTR; n = 5 for all except for NT-4/5 [n = 4]) or from patients with mastocytosis (MAST; n = 5 including CM [n = 1] and ISM [n = 4] for each group except for NT-4/5 [n = 3]) are depicted. Mean values ± SEMs are shown.
Elevated NGF, NT-3, TrkB, and TrkC mRNA expression on SMCs from patients with mastocytosis. Relative mRNA expression of NGF-β (A), NT-3 (B), NT-4/5 (C), TrkA (D), TrkB (E), and TrkC (F) on cultured SMCs isolated from healthy skin (CTR; n = 5 for all except for NT-4/5 [n = 4]) or from patients with mastocytosis (MAST; n = 5 including CM [n = 1] and ISM [n = 4] for each group except for NT-4/5 [n = 3]) are depicted. Mean values ± SEMs are shown.
Increased infiltration of MCs expressing TrkA, TrkB, and TrkC in the dermis and of MCs expressing TrkA and TrkC in the gut of patients with mastocytosis
Increased infiltration of MCs in the dermis of patients with mastocytosis was demonstrated by toluidine blue staining of lesional skin (Figure 3A-C). Immunohistochemical staining of those skin sections revealed significantly higher numbers of dermal cells expressing TrkA, TrkB, and TrkC in the patients compared with the control participants (Figure 3D-L).
Increased number of MCs correlates with upregulated number of TrkA+, TrkB+, and TrkC+cells infiltrating the dermis in CM. Toluidine blue (A-C), anti-TrkA (D-F), anti-TrkB (G-I), and anti-TrkC (J-L) staining of skin biopsies of healthy control participants (CTR) and patients with mastocytosis (MAST; lesional skin). Immunohistochemistry stainings of a representative paraffin section of healthy skin (A, D, G, J), CM (B, E, H, K), and mean values and SEMs of the number of cells in the upper (left side) and lower (right side) dermis of 10 control participants and 10 patients with mastocytosis (C, F, I, L: n = 5 for CM; n = 4 for ISM; and n = 1 for SM-AHNMD) are depicted.
Increased number of MCs correlates with upregulated number of TrkA+, TrkB+, and TrkC+cells infiltrating the dermis in CM. Toluidine blue (A-C), anti-TrkA (D-F), anti-TrkB (G-I), and anti-TrkC (J-L) staining of skin biopsies of healthy control participants (CTR) and patients with mastocytosis (MAST; lesional skin). Immunohistochemistry stainings of a representative paraffin section of healthy skin (A, D, G, J), CM (B, E, H, K), and mean values and SEMs of the number of cells in the upper (left side) and lower (right side) dermis of 10 control participants and 10 patients with mastocytosis (C, F, I, L: n = 5 for CM; n = 4 for ISM; and n = 1 for SM-AHNMD) are depicted.
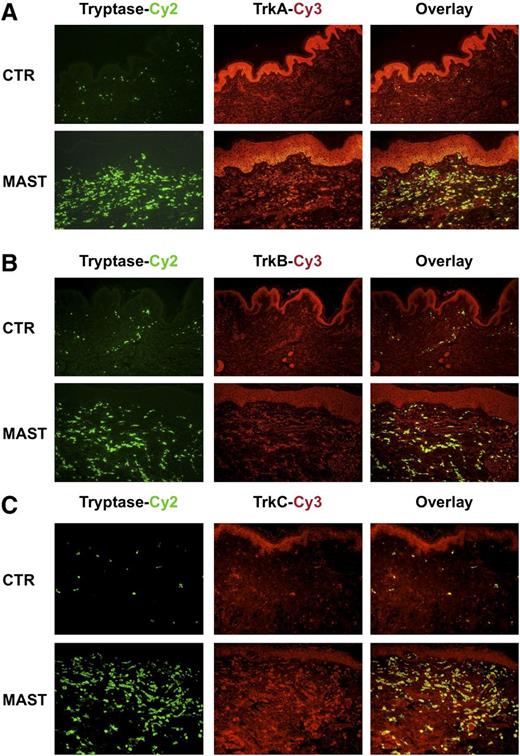
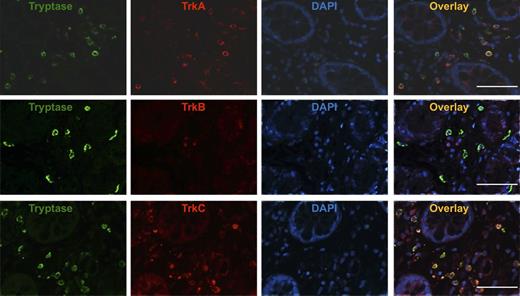
To determine whether the TrkA+, TrkB+, and TrkC+ cells are MCs, we performed immunofluorescence double staining. TrkA+, TrkB+, and TrkC+ cells in patients with mastocytosis spatially overlaid with cells expressing tryptase, demonstrating that most of the cells expressing Trk receptors in the dermis were tryptase+ SMCs (Figure 4A-C). Furthermore, TrkA- and TrkC-positive, but TrkB-negative, tryptase+ MCs were detectable in the gut (Figure 5) of patients with SM. Taken together, immunofluorescence double staining confirmed infiltration of the skin with TrkA+, TrkB+, and TrkC+ tryptase+ MCs and gut with TrkA+, TrkB-, and TrkC+ tryptase+ MCs.
Immunofluorescence double staining identifies TrkA+, TrkB+, and TrkC+cells infiltrating the skin of patients with mastocytosis as MCs. (A-C) Representative immunofluorescence double staining of skin biopsy taken from a healthy control patient (CTR) and a patient with mastocytosis (MAST) with an anti-tryptase antibody labeled with Cy-2 (green) and anti-TrkA, anti-TrkB, and anti-TrkC antibodies labeled with Cy-3 (red) and the overlay pictures of both stainings are shown.
Immunofluorescence double staining identifies TrkA+, TrkB+, and TrkC+cells infiltrating the skin of patients with mastocytosis as MCs. (A-C) Representative immunofluorescence double staining of skin biopsy taken from a healthy control patient (CTR) and a patient with mastocytosis (MAST) with an anti-tryptase antibody labeled with Cy-2 (green) and anti-TrkA, anti-TrkB, and anti-TrkC antibodies labeled with Cy-3 (red) and the overlay pictures of both stainings are shown.
TrkA+and TrkC+MCs infiltrate the gut of patients with SM. Representative immunofluorescence double staining of gut sections (original magnification ×400) of patients with mastocytosis (MAST; n = 4) with an anti-tryptase antibody labeled with Cy-2 (green) and anti-TrkA, anti-TrkB, and anti-TrkC antibodies labeled with Cy-3 (red) overlay pictures of both stainings are shown. White bar = 50 µm.
TrkA+and TrkC+MCs infiltrate the gut of patients with SM. Representative immunofluorescence double staining of gut sections (original magnification ×400) of patients with mastocytosis (MAST; n = 4) with an anti-tryptase antibody labeled with Cy-2 (green) and anti-TrkA, anti-TrkB, and anti-TrkC antibodies labeled with Cy-3 (red) overlay pictures of both stainings are shown. White bar = 50 µm.
Enhanced expression of receptor isoforms of TrkB and TrkC on human SMCs from patients with mastocytosis
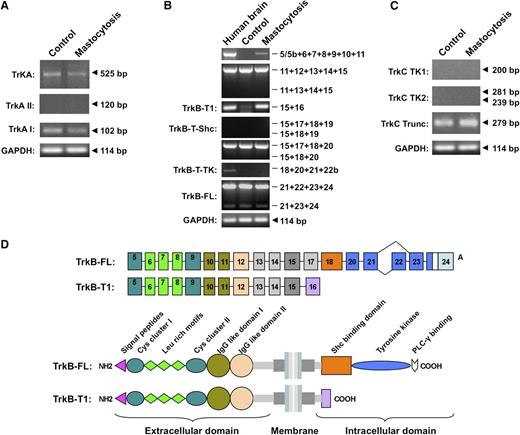
Human SMCs from both patients with mastocytosis and control participants express TrkA mRNA, which contains highly conserved regions of the extracellular domain as well as the tyrosine kinase domain (Figure 6A), suggesting functional TrkA receptors. Human SMCs expressed exclusively the exon lacking the TrkAI splice variant (Figure 6A, 102bp), which is supposed to be less responsive to NT-3 than TrkAII.9 Both FL and truncated TrkB mRNA were expressed in SMCs from patients with mastocytosis. Sequence determination confirmed expression of the TrkB isoforms (data not shown). Patients with mastocytosis had a different gene expression profile of TrkB on human SMCs compared with control participants. Exons 1 through 4 and a major part of exon 5 encode the conventional 5′ untranslated region (UTR) of the human TrkB gene and serve as transcription start sites. We detected higher expression of the 5′- UTR gene in the SMCs of patients with mastocytosis (supplemental Figure 1). SMCs from patients and control participants had the same mRNA expression level of exon 24, which encodes the FL-TrkB receptor containing the tyrosine kinase domain (Figure 6B, exons 21-24). PCR results indicated that both FL-TrkB and a receptor variant without exon 22 are expressed by human SMCs.
Expression patterns of Trk receptors on human SMCs from patients with mastocytosis and healthy control participants. (A) FL TrkA and alternatively spliced trkA mRNA isoforms in human SMCs. (B) Expression of TrkB transcripts in human SMCs. Amplified exons of the TrkB receptor are depicted on the right side, and names of alternative TrkB isoforms are shown on the left. (C) Expression of tyrosine kinase domain (TK1: exons 13-14; TK2: exons 15-17) and truncated isoforms of TrkC in human SMCs. Representative PCR results of cDNAs from SMCs of patients with mastocytosis (MAST, n = 13: n = 5 for CM; n = 7 for ISM; and n = 1 for SM-AHNMD) and healthy individuals (CTR; n = 14) are shown. (D) Schematic drawing of TrkB-FL and TrkB-T1 mRNA and protein isoforms expressed on human SMCs. Exons are shown as boxes, and introns are shown as lines. C, cysteine-rich region; Leu rich, leucine-rich region; IG like, immunoglobulin-like domain; SHC, Shc-binding domain; and PLC-γ, PLC-γ–binding domain.
Expression patterns of Trk receptors on human SMCs from patients with mastocytosis and healthy control participants. (A) FL TrkA and alternatively spliced trkA mRNA isoforms in human SMCs. (B) Expression of TrkB transcripts in human SMCs. Amplified exons of the TrkB receptor are depicted on the right side, and names of alternative TrkB isoforms are shown on the left. (C) Expression of tyrosine kinase domain (TK1: exons 13-14; TK2: exons 15-17) and truncated isoforms of TrkC in human SMCs. Representative PCR results of cDNAs from SMCs of patients with mastocytosis (MAST, n = 13: n = 5 for CM; n = 7 for ISM; and n = 1 for SM-AHNMD) and healthy individuals (CTR; n = 14) are shown. (D) Schematic drawing of TrkB-FL and TrkB-T1 mRNA and protein isoforms expressed on human SMCs. Exons are shown as boxes, and introns are shown as lines. C, cysteine-rich region; Leu rich, leucine-rich region; IG like, immunoglobulin-like domain; SHC, Shc-binding domain; and PLC-γ, PLC-γ–binding domain.
TrkB-T1-mRNA expression on SMCs from patients with mastocytosis was increased compared with control participants (Figure 6B, exons 15-16). Other truncated TrkB isoforms, such as TrkB-TK and TrkB-T-Shc using exon 22b and exon 19, respectively, were not detected. Moreover, a recently identified TrkB receptor using exon 5c12 was not expressed on human SMCs. It is noteworthy that SMCs from the patients had a higher mRNA expression of extracellular domain of TrkB than the control participants (Figure 6B, exons 5-11). A schematic picture of TrkB-FL and TrkB-T1 isoforms identified on human SMCs according to TrkB PCR data illustrates expression of TrkB on human SMCs (Figure 6D). Finally, SMCs of both patients with mastocytosis and control participants expressed the truncated TrkC isoforms lacking the tyrosine kinase domain (Figure 6C).
NGF-β increases the migratory properties of peripheral CD117+ cells via TrkA
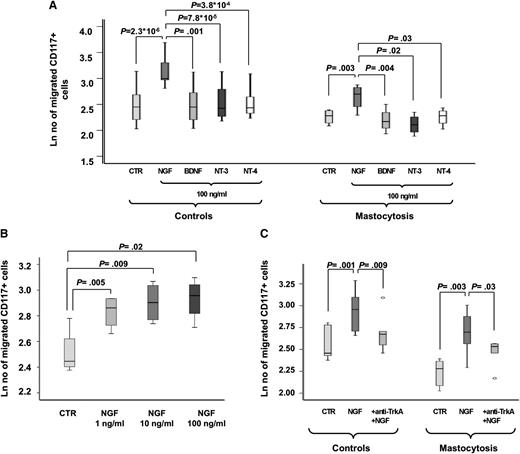
Migration of CD117+ progenitor cells from the blood of both patients with mastocytosis (P = .003) and control participants (P = 2.3*10−6) was significantly increased toward NGF-β (P = 1.7*10−5 for all participants), but not toward a BDNF, NT-3, and NT-4 gradient (Figure 7A) and did not differ significantly between the patients and the control participants. Migration toward NGF-β was significant higher compared with migration to BDNF, NT-3, and NT-4 in both the patients (P = .004; P = .02; and P =.03, respectively) and the control participants (P = .001; P = 7.8*10−5; and P = 3.8*10−4, respectively). The number of migrated cells increased in a dose-dependent fashion (Figure 7B). The addition of anti-human TrkA antibody specifically blocking cell-surface TrkA-mediated activity significantly prevented migration of CD117+ cells toward NGF-β in patients with mastocytosis (P = .03) and in control participants (P = .009) (P = .002 for all participants), indicating that NGF-β–driven migration of CD117+ cells was mainly mediated via TrkA (Figure 7C).
NGF-β–enhanced migratory properties of CD117+cells from the blood. (A) Relative change of the migratory activity of human peripheral CD117+ progenitor cells from healthy control participants (CTR; n = 12 for NGF; n = 6 for BDNF, NT-3, and NT-4) and patients with mastocytosis (MAST; n = 6; n = 1 for CM, n = 4 for ISM, and n = 1 for SSM) toward a NGF-β, BDNF, NT-3, and NT-4 gradient in transwell chamber experiments compared with unstimulated control participants is shown as mean values and SEMs. (B) Number of human peripheral migrated CD117+ progenitor cells from healthy control participants (n = 4) toward a NGF-β gradient increases in a dose-dependent fashion. (C) Addition of anti-human TrkA antibody significantly prevented migration of CD117+ cells toward NGF-β in both patients with mastocytosis and healthy control participants (n = 6 each), indicating that NGF-β–driven migration of CD117+ cells was mainly mediated via TrkA.
NGF-β–enhanced migratory properties of CD117+cells from the blood. (A) Relative change of the migratory activity of human peripheral CD117+ progenitor cells from healthy control participants (CTR; n = 12 for NGF; n = 6 for BDNF, NT-3, and NT-4) and patients with mastocytosis (MAST; n = 6; n = 1 for CM, n = 4 for ISM, and n = 1 for SSM) toward a NGF-β, BDNF, NT-3, and NT-4 gradient in transwell chamber experiments compared with unstimulated control participants is shown as mean values and SEMs. (B) Number of human peripheral migrated CD117+ progenitor cells from healthy control participants (n = 4) toward a NGF-β gradient increases in a dose-dependent fashion. (C) Addition of anti-human TrkA antibody significantly prevented migration of CD117+ cells toward NGF-β in both patients with mastocytosis and healthy control participants (n = 6 each), indicating that NGF-β–driven migration of CD117+ cells was mainly mediated via TrkA.
Discussion
Here we report for the first time the expression of TrkA, TrkB, and TrkC on human SMCs and an increased expression on SMCs in patients with mastocytosis. Intestinal MCs from these patients expressed TrkA and TrkC, but not TrkB. We observed a significant increase of cells expressing the NGF high-affinity receptor TrkA in affected skin lesions of patients with mastocytosis, which could be identified as MCs by immunofluorescence staining and flow cytometry. Human SMCs from both patients and control participants expressed TrkA mRNA, which contains highly conserved regions of the extracellular domain as well as the tyrosine kinase domain, suggesting functional TrkA receptors.
Our results indicate that human SMCs express exclusively the TrkAI splice variant, but not TrkAII. These results, together with (i) increased NGF and NT-3 mRNA expression in SMCs; and (ii) the correlation of NGF-β and NT-4 serum levels with serum tryptase, strongly suggest that MCs themselves might serve as a source for the elevated circulating NT serum levels observable in patients with mastocytosis.
Previously, NTs have also been shown to exert chemotactic effects on fibroblasts,38 NGF on RPMCs,25,26 and melanocytes.39 In line with those findings, we demonstrated a significantly increased migration of peripheral CD117+ cells toward an NGF-β gradient via TrkA, which was expressed on the MCs of the skin and gut, organs frequently infiltrated in mastocytosis. The expression of TrkA and TrkC on human intestinal MCs is consistent with a previous report, which observed TrkA and TrkC expression on the mRNA and protein level,24 but demonstration of TrkA, TrkB, and TrkC on SMCs in general and of NT receptors in mastocytosis is novel.
Most interestingly, the human leukemia MC line-1 (HMC-1) has been shown to express TrkA, TrkB, and TrkC receptor proteins containing FL tyrosine kinase domains, as well as TrkAI and truncated TrkB and TrKC.9 NGF has been shown to induce upregulation of early growth response genes and other proliferation and survival signals in HMC-1 via TrkA9,18-20 and rescue HMC-1 (V560G c-kit) cells from cell death mediated by imatinib mesylate. NGF, together with SCF,23 could also promote growth, differentiation, and survival of cord blood–derived MCs9,19,20,23 and murine MCs.25,41 Thus, increased NGF serum levels, together with elevated expression of NGF and TrkA on SMCs in patients with mastocytosis and an increased migration of CD117+ cells from the blood toward an NGF gradient via TrkA, might contribute to the increased numbers of MCs via increased migration and proliferation of MC progenitors.
As also several other skin cells such as fibroblasts, keratinocytes, melanocytes, lymphocytes, macrophages, endothelial cells, eosinophils, and cutaneous nerve fibers have been shown to produce NTs,16,38-41 the skin represents an environment rich in NTs that might attract the migration of MC progenitor cells. A higher level of NGF-β in the sera of patients with IBMM without skin lesions might be of pathogenic significance, as it would create less of a gradient between the skin and intravascular compartment and discourage MC progenitors from migrating to the skin. However, the number of patients with IBMM was too small to allow further conclusions, and there was no significant difference in NGF serum levels between patients with CM or MIS compared with those with IBMM.
Furthermore, we detected a significantly higher expression of TrkB and TrkC on the SMCs of patients with mastocytosis both on the mRNA and protein levels, as well as increased levels of ligands NT-3 and NT-4 in these patients. TrkB receptors exist as an FL isoform (FL-TrkB) and as 3 different truncated isoforms (TrkB-T1, TrkB-T-TK, and TrkB-Shc).9-12 Previously, the expression of TrkAI, truncated TrkB, and truncated TrkC could be shown on HMC-1 cells.9 Our PCR results demonstrated that human SMCs express both FL-TrkB and truncated TrkB-T1, but not TrkB-T-TK and TrkB-T-Shc. Higher expression of the 5′- UTR gene in the SMCs of patients with mastocytosis suggests higher activity of gene transcription. It is interesting to note that the SMCs from patients with mastocytosis vs control participants had much higher expression levels of truncated TrkB-T1 and genes encoding the extracellular domain of TrkB, whereas transcription of the intracellular domain of the FL-TrkB was not affected. Because both FL and truncated receptors need the extracellular domain to form the complete receptor, less expression of the extracellular domain in human SMCs could be the limiting step for transcription of both FL-TrkB and truncated TrkB-T1. The biological functions of FL-TrkB and truncated TrkB and their cooperation on human MCs are still largely unknown. Studies investigating the development of the nervous system and tumorigenesis indicate an essential role of Trk receptors for cell proliferation and tissue development. Patients with tumors that have high expression of TrkB are regarded to have a poor prognosis.13,42 FL-TrkB is preferentially expressed during the early stages of embryogenesis and is replaced by truncated TrkB-T1 during postnatal development.43,44 The truncated TrkB-T1 consists of a transmembrane and extracellular domain, which binds its natural ligands, whereas its short intracellular tail does not have any tyrosine kinase activity. TrkB-T1 has long been regarded as a negative regulator of BDNF signaling via forming heterodimers with FL-TrkB.45 However, recent studies have indicated that TrkB-T1 is a functional receptor that is capable of mediating intracellular signaling cascades.46,47 Here, the identification of FL-TrkB expression and elevated expression of truncated TrkB-T1 on SMCs in patients with mastocytosis indicates an abnormal status of TrkB expression in this disease, and the putative pathophysiological role of TrkB has to be elucidated.
TrkA and TrkC have recently been demonstrated to act as dependence receptors, which induce signals for survival, differentiation, and migration in the presence of their ligand and apoptosis in the absence of their ligands NGF-β and NT-3.42 For both ligands, we could show increased circulating levels as well as increased expression on SMCs in patients with mastocytosis.
In human intestinal MCs, NT-3, but not NGF, has been shown to promote proliferation and reduce apoptosis of human intestinal MCs via TrkC, together with SCF.24 Conversely, increased numbers of MCs have been observed in the neonatal skin of NT-3–overexpressing mice, without showing a higher rate of MC proliferation.48 However, the expression and, presumably, functions of NTs and their high-affinity receptors for NTs differ between MCs from humans and mice and also between different human tissues and human cell lines.9,14,15,18-26,48
In our study, we could demonstrate for the first time significantly higher circulating levels of the NTs NGF-β, NT-3, and NT-4 and increased migration of CD117+ cells from the blood toward an NGF gradient via TrkA. NTs might contribute to the augmented MC tissue infiltration in mastocytosis via increased migration of MC progenitor cells toward NGF and/or stimulation of MC differentiation and proliferation by NGF and NT-3. As MCs themselves represent a source of NTs with functional receptors, they might augment these effects in an autocrine feedback loop.
In conclusion, our data indicate a pivotal role of NTs in the pathophysiology of mastocytosis. The sophisticated network between MC progenitors in the blood, MCs in the skin, and soluble mediators of the nervous system such as NTs might be of major relevance in understanding the mechanisms that trigger mastocytosis and other MC-driven diseases. Furthermore, better knowledge about factors promoting MC infiltration might help to identify structures, which could be efficiently targeted in therapeutic approaches, aimed at attenuating unwanted overactivation and tissue infiltration of MCs. Several Trk inhibitors are currently being developed with some small-molecule inhibitors already being in phase 1 and 2 clinical trials,13,42 which might potentially also present a therapeutic option for mastocytosis in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Dr Molderings, Institute of Pharmacology and Toxicology, University of Bonn, for critically reviewing the manuscript and Prof Dr A. Becker, Department of Neuropathology, University of Bonn, for providing human brain tissue as a control. We thank Nils Schoof and Mareike Borstar for excellent technical support. We thank all of the patients for their willingness to participate in this study.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB704 TPA4) and a BONFOR grant of the University of Bonn. This work was also supported by a Heisenberg professorship of the DFG NO454/5-1 (N.N.), and a BONFOR Gerok fellowship and a young investigator fellowship of the University of Bonn (L.M.). N.N. is a member of the Cluster of Excellence ImmunoSensation of the German Research Council.
Authorship
Contributions: W.-M.P., L.M., and N.N. performed study concept and design; W.-M.P., L.M., J.P.A., I.G., J.K., E.W., and N.N. acquired the data; L.M., J.-P.A., S.P., J.O., and K.W. recruited the patients; W.-M.P., L.M., J.-P.A., I.G., J.K., R.F., and N.N. analyzed and interpreted the data; W.-M.P., L.M., J.-P.A., and N.N. drafted the manuscript; U.R., I.G., J.K., E.W., S.P., W.Z., J.O., R.F., K.W., and L.B.S. performed critical revision of the manuscript for important intellectual content; L.M. and N.N. obtained funding.
Conflict-of-interest disclosure: The authors declare no conflict of interest.
Correspondence: Natalija Novak, Department of Dermatology and Allergy, University of Bonn, Sigmund-Freud-Str. 25, 53127 Bonn, Germany; e-mail: Natalija.Novak@ukb.uni-bonn.de.
References
Author notes
W.-M.P., L.M., and J.-P.A. contributed equally to this study.

![Figure 2. Elevated NGF, NT-3, TrkB, and TrkC mRNA expression on SMCs from patients with mastocytosis. Relative mRNA expression of NGF-β (A), NT-3 (B), NT-4/5 (C), TrkA (D), TrkB (E), and TrkC (F) on cultured SMCs isolated from healthy skin (CTR; n = 5 for all except for NT-4/5 [n = 4]) or from patients with mastocytosis (MAST; n = 5 including CM [n = 1] and ISM [n = 4] for each group except for NT-4/5 [n = 3]) are depicted. Mean values ± SEMs are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/10/10.1182_blood-2012-12-469882/4/m_1779f2.jpeg?Expires=1769169495&Signature=w9Ig9ViwLiK93Pcuw3S9j~5Z0-y7PeRsh~W4nEJm8z~1pFUzcV3PKKU6tQUpe5CL6cgE4wScNMvV-nGVeUGKxVvKPAH4RqiI0rAf16SX32f5XkaedzeoriN8QN0MjJIxP7oasUj6M5aenMUFK8bXFRiF5G4Rm6Fw57KhXN5iQJMGcN54zsC3XmB6BlCsDHr37rs5PKpM7JLocCukCpLLOkIg62UQJd4-AuxQaVhYZTQyA-kuLguLDhSrFZa02VXVUyZqL3TCxpi36LxjBXQT51v07prbSlvfeMKbiWSQ17sZ2R9XzPfzaaSJFsc3aYTEA1jujaZUMeCkqlWsf5Zg9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)