Key Points
TSP-1 binding to CD36 recruits SHP-1 to CD36-VEGFR2 complex in microvascular endothelial cells.
SHP-1 recruitment to CD36-VEGFR2 complex dephosphorylates VEGFR2 and inhibits angiogenesis.
Abstract
Thrombospondin-1 (TSP-1) inhibits growth factor signaling at the receptor level in microvascular endothelial cells (MVEC), and CD36 has been suggested to be involved in this inhibition, but the mechanisms are not known. We hypothesized that CD36-TSP-1 interaction recruits Src homology 2 domain–containing protein tyrosine phosphatase (SHP)-1 to the vascular endothelial growth factor receptor 2 (VEGFR2) signaling complex and attenuates vascular endothelial growth factor (VEGF) signaling. Western blots of anti-CD36 and anti-VEGFR2 immunoprecipitates from VEGF-treated MVEC showed that exposure of the cells to a recombinant protein containing the CD36 binding domain of thrombospondin-1 (known as the TSR domain) induced association of SHP-1 with the VEGFR2/CD36 signaling complex and thereby suppressed VEGFR2 phosphorylation. SHP-1 phosphatase activity was increased in immunoprecipitated VEGFR2 complexes from TSR-treated cells. Silencing CD36 expression in MVEC by small interfering RNA (siRNA) or genetic deletion of cd36 in mice showed that TSR-induced SHP-1/VEGFR2 complex formation required CD36 in vitro and in vivo. Silencing SHP-1 expression in MVEC by siRNA abrogated TSR-mediated inhibition of VEGFR2 phosphorylation as well as TSR-mediated inhibition of VEGF-induced endothelial cell migration and tube formation. These studies reveal a SHP-1–mediated antiangiogenic pathway induced by CD36-TSP-1 interaction that inhibits VEGFR2 signaling and they provide a novel target to modulate angiogenesis therapeutically.
Introduction
CD36 is a multiligand scavenger receptor expressed on the surface of platelets, MVEC, mononuclear phagocytes, adipocytes, hepatocytes, myocytes, and some epithelia.1 It was first identified as glycoprotein IV on platelets.2 On MVEC, CD36 is a receptor for TSP-1 and related proteins containing the so-called “thrombospondin type I structural homology domain (TSR).” It functions as a negative regulator of angiogenesis1,3-5 and therefore plays a role in tumor growth, inflammation, wound healing, and other pathological processes requiring neovascularization.6,7 Binding of TSP-1 or TSR proteins to CD36 inhibits growth factor–induced proangiogenic signals that mediate endothelial cell proliferation, migration, and tube formation, and instead generates antiangiogenic signals that lead to apoptosis.4,8 In vivo, CD36 null mice exhibit an increase in vessel density in the brain, an organ in which early angiogenesis is modulated by high levels of TSP-1, increased tumor angiogenesis in subcutaneously injected TSP-expressing tumor cells, and a lack of response to antiangiogenic effect of TSP-1 in in vivo angiogenesis assays.8,9
It was recently shown that TSP-1 also inhibits growth factor signaling at the vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) receptor level in endothelial cells and a mouse model.10-12 In these studies, VEGF-induced VEGFR2 phosphorylation and downstream Akt signaling were inhibited by pretreatment of cells with recombinant TSR. The studies showed that CD36 and VEGFR2 form a complex in MVEC, therefore suggesting that VEGFR2 dephosphorylation could be mediated by CD36. No direct evidence demonstrated a CD36 requirement in this inhibition, and the molecular mechanisms remain unclear.
CD36 modulates cell signaling in some cases by interacting with other receptors on the cell surface, including integrins,11,13,14 tetraspanins,13 and Toll-like receptors.15,16 The 2 cytoplasmic domains of CD36 are very short, with no intrinsic kinase or phosphatase activity, but the carboxyl terminal domain has been shown to form a complex with intracellular signaling molecules, including Src family kinases and upstream mitogen-activated protein kinases.1,8,17
Of the 3 known VEGF receptors, VEGFR2 is the primary mediator of angiogenic signaling.18 There are several tyrosine residues on VEGFR2 that become phosphorylated upon VEGF exposure. Among these, Tyr1175 is the most important in angiogenesis.19 Phosphorylation of VEGFR2 is regulated by members of the SHP family.20-22 There are 2 members in this cytoplasmic phosphatase family: SHP-1 and SHP-2. Dephosphorylation of VEGFR2 Tyr1175 is mediated by SHP-1, but not by SHP-2.21,23 Knockdown of SHP-1 by small interfering RNA (siRNA) promotes VEGF-mediated DNA synthesis in human umbilical vein endothelial cells and accelerates angiogenesis in a rat model.20,21
Interestingly, our group recently showed that SHP-2 interacts with CD36 in macrophages and that its activity was modulated by the binding of oxidized low-density lipoprotein to CD36.24 Whether SHP-1 interacts with CD36 is not known. In this paper, we used in vitro and in vivo assays to define a mechanism by which TSP-1 or recombinant TSR peptide suppresses VEGF signaling in MVEC via CD36 by regulating SHP-1 localization and activity. We found that TSR induced the association of SHP-1 with both CD36 and VEGFR2, and that in the presence of TSR, the addition of VEGF dramatically increased phosphatase activity associated with VEGFR2. Using siRNA gene silencing in cultured MVEC and genetic deletion in mice, we showed that CD36 was required for TSR-induced SHP-1/VEGFR2 complex formation and for TSR-mediated VEGFR2 dephosphorylation. By using SHP-1 siRNA and a pharmacologic inhibitor, we also showed that SHP-1 was required for dephosphorylation of VEGFR2, inhibition of VEGF-induced cell migration, and in vitro tube formation by TSR. These studies establish that recruitment of SHP-1 to CD36-VEGFR2 complexes by CD36 interaction with TSP-1 plays a key role in CD36 antiangiogenic signaling in MVEC that is different from the previously well-described proapoptotic pathways mediated by Fyn and p38 MAP kinases. This study adds to the current understanding of the role of CD36 in angiogenesis and provides new insight into modulating angiogenesis.
Methods
Immunofluorescence studies
MVEC grown on 12-well plates to 60% to 80% confluency were treated with 10 nmol/L TSR in low-serum medium for 4 hours and then exposed to 50 ng/mL VEGF for 5 minutes. Cells were then washed in phosphate-buffered saline, fixed with 4% paraformaldehyde, and permeabilized with Triton X-100 (Sigma-Aldrich) before probing with anti-CD36 and anti–SHP-1 primary antibodies raised in rabbit and mouse, respectively. Anti-rabbit and anti-mouse immunoglobulin G (IgG) antibodies labeled, respectively, with Alexa Fluor dyes 594 and 488 (Invitrogen) were used to immunostain CD36 and SHP-1. Images were obtained at room temperature with a laser scanning confocal microscope (Olympus FV1000) using a 60× objective with Fluoview software (Olympus).
In vitro phosphatase activity assay
MVEC were pretreated with 10 nmol/L TSR before exposure to VEGF for 15 minutes. Cells were lysed with lysis buffer, and VEGFR2 complexes were immunoprecipitated as described in the supplemental Data (available on the Blood website) and then resuspended in 1 mol/L diethanolamine at a pH of 9.8. Phosphatase substrate, p-nitrophenyl phosphate disodium salt (pNPP 1 mg/mL; Thermo Scientific), was added and incubated for 1 hour at room temperature.21 The reaction was stopped by the addition of 2 mol/L NaOH. Phosphatase activity was detected as absorbance at 405 nm using a SpectraMax 190 Microplate Reader (Molecular Devices).
Cell migration assay
Migration of MVEC was measured in a modified Boyden Chamber assay using Transwell inserts with an 8.0-μm pore polycarbonate membrane. siRNA knock-down or inhibitor (10 µmol/L)-treated MVEC were allowed to migrate for 15 hours toward VEGF (50 ng/mL) in the lower chamber. In some cases, the cells were also exposed to 10 nmol/L TSR. Migrated cells were fixed with 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole. The number of cells migrated were counted from random fields under fluorescence microscopy and the results expressed as the percent of cells migrated compared with the basal migration in wells with no treatment within each group.
In vitro endothelial tube formation assay
MVEC were transfected with CD36 or control siRNA as described before and then incubated with 10 nmol/L TSR in serum-free medium at 37°C for 4 hours. Cells were then trypsinized, resuspended in serum-free EBM-2 MV medium, and added to wells in a 24-well plate (105 cells/well) that were previously coated with Matrigel (BD Biosciences). Recombinant 5VEGF (50 ng/mL) with or without the SHP inhibitor NSC-87877 (100 μmol/L) was added to some wells. Cells were incubated at 37°C for 16 hours and then fixed with 4% paraformaldehyde and stained with fluorescence-labeled phalloidin (Invitrogen). Fluorescence images were taken from 4 randomly chosen areas and tube formation analyzed by ImageJ software (National Institutes of Health), as previously described.6
In vivo VEGFR2 phosphorylation assay
C57BL/6 wild-type and cd36 null mice25 were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and then injected with 1 μg VEGF in 100 μL saline or saline alone via the jugular vein. Lung tissue was harvested 5 minutes after injection, minced in liquid nitrogen, and then lysed in 1 mL buffer, as for immunoprecipitation, for 5 mintues. Lysates were centrifuged at 12 000g for 5 minutes to remove cell debris and then analyzed by western blot with antibodies to VEGFR2, pTyr1175 VEGFR2, CD36, and tubulin. Lysates were also incubated with anti-VEGFR2 antibodies to precipitate VEGFR2, and the precipitates were analyzed by western blot with antibodies to VEGFR2, CD36, and SHP-1. All procedures on animals were approved by the Cleveland Clinic and Medical College of Wisconsin Institutional Animal Care and Use Committees. Mice were housed in facilities fully accredited by AALAC and in accordance with all federal and local regulations.
Statistics
Student t test and one sample t test were used where appropriate. A P value < .05 was considered significant. Data are expressed as mean ± standard deviation.
Results
TSR induces association of active SHP-1 with CD36 and VEGFR2 in MVEC
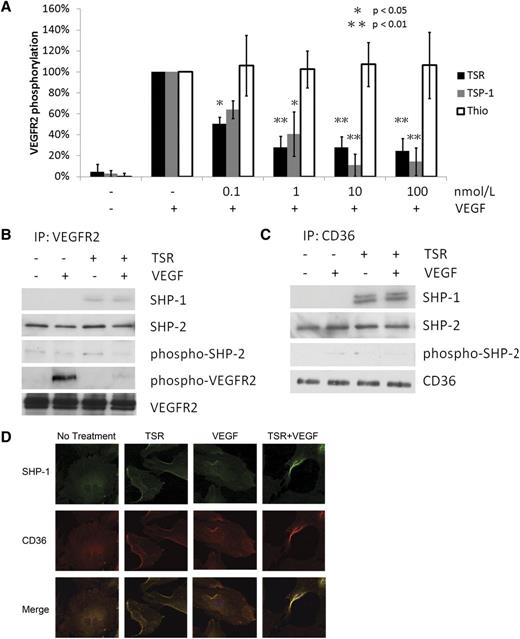
To verify that TSP-1 inhibits VEGF signaling at the VEGFR2 receptor level, MVEC pretreated with TSP-1 for 4 hours were exposed to 50 ng/mL VEGF for 5 minutes. Because TSP-1 is an endogenous protein, to mimic the in vivo prolonged exposure to TSP-1, we treated the cells for 4 hours with TSP-1 or TSR. As shown in Figure 1A-B, VEGFR2 Tyr1175 phosphorylation levels were very low or undetectable in untreated cells but increased significantly in those exposed to VEGF. Pretreatment with TSP-1 decreased VEGF-induced VEGFR2 phosphorylation in a concentration-dependent manner with 0.1 nmol/L and 100 nmol/L TSP-1 inhibiting phosphorylation by 40% and 80%, respectively. We also confirmed the previously reported association of CD36 with VEGFR2 by coimmunoprecipitation in early passage (<6) MVEC but not in late passage MVEC (>9) that retain VEGFR2 but lose CD36 expression (supplemental Figure 2). TSP-1 is a large multidomain protein that interacts with many receptors on the cell membrane. To determine the specific role of CD36, we showed that a recombinant TSR peptide behaved similarly to intact TSP-1 (Figure 1A; supplemental Figure 1). Because the recombinant TSR was produced as a thioredoxin fusion protein, the thioredoxin tag, without the recombinant TSR protein, was used as a negative control and it showed no effect on VEGFR2 phosphorylation.
TSR induces SHP-1 association with CD36 and VEGFR2 in MVEC. (A) MVEC were cultured to confluence and then treated with TSP-1, recombinant TSR, or thioredoxin (Thio) control for 4 hours before exposure to 50 ng/mL VEGF for 5 minutes. VEGFR2 phosphorylation was analyzed by western blot with an antibody to VEGFR2 pTyr1175. Blots were reprobed with anti-VEGFR2 as a loading control. VEGFR2 phosphorylation levels are expressed as the ratio of phosphorylated VEGFR2 to total VEGFR2. (B-C) MVEC were treated with TSR (10 nmol/L) in low-serum medium for 4 hours before exposure to VEGF (50 ng/mL) for 15 minutes. Cell lysates were then immunoprecipitated with anti-VEGFR2 (B) or anti-CD36 (C), and coprecipitated proteins were detected by western blot analysis with antibodies to SHP-1, SHP-2, phospho-SHP-2, phospho-VEGFR2, VEGFR2, or CD36. Blots are representative of 3 experiments. (D) Immunofluorescence images of MVEC treated with 10 nmol/L TSR and/or 50 ng/mL VEGF and then probed with mouse monoclonal anti–SHP-1 and rabbit polyclonal anti-CD36, followed by Alexa Fluor 488–conjugated anti-mouse IgG and Alexa Fluor 594–conjugated anti-rabbit IgG. The top row shows Alexa Fluor 488 images, the middle row shows Alexa Fluor 594 images, and the bottom row shows the merged images.
TSR induces SHP-1 association with CD36 and VEGFR2 in MVEC. (A) MVEC were cultured to confluence and then treated with TSP-1, recombinant TSR, or thioredoxin (Thio) control for 4 hours before exposure to 50 ng/mL VEGF for 5 minutes. VEGFR2 phosphorylation was analyzed by western blot with an antibody to VEGFR2 pTyr1175. Blots were reprobed with anti-VEGFR2 as a loading control. VEGFR2 phosphorylation levels are expressed as the ratio of phosphorylated VEGFR2 to total VEGFR2. (B-C) MVEC were treated with TSR (10 nmol/L) in low-serum medium for 4 hours before exposure to VEGF (50 ng/mL) for 15 minutes. Cell lysates were then immunoprecipitated with anti-VEGFR2 (B) or anti-CD36 (C), and coprecipitated proteins were detected by western blot analysis with antibodies to SHP-1, SHP-2, phospho-SHP-2, phospho-VEGFR2, VEGFR2, or CD36. Blots are representative of 3 experiments. (D) Immunofluorescence images of MVEC treated with 10 nmol/L TSR and/or 50 ng/mL VEGF and then probed with mouse monoclonal anti–SHP-1 and rabbit polyclonal anti-CD36, followed by Alexa Fluor 488–conjugated anti-mouse IgG and Alexa Fluor 594–conjugated anti-rabbit IgG. The top row shows Alexa Fluor 488 images, the middle row shows Alexa Fluor 594 images, and the bottom row shows the merged images.
Phosphorylation of VEGFR2 is regulated by the SHP family of phosphatases. The 2 members of this family, SHP-1 and SHP-2, dephosphorylate different tyrosine residues on the receptor. To determine the potential role of these phosphatases in TSR-mediated inhibition of VEGFR signaling, the association of SHP-1 and SHP-2 with VEGFR2 and CD36 was assessed by immunoprecipitation and western blot analysis. SHP-2 was coprecipitated by both anti-VEGFR2 (Figure 1B) and anti-CD36 (Figure 1C) in untreated cells, but treatment with VEGF, TSR, or both had no effect. The activity of CD36-associated SHP-2, as determined by western blot analysis of the level of phospho–SHP-2, was low and was also not influenced by VEGF or TSR treatment. SHP-1, in contrast to SHP-2, was precipitated from the cells by anti-VEGFR2 (Figure 1B) and anti-CD36 (Figure 1C) only after treatment with TSR. VEGF alone did not induce SHP-1 association with CD36 or VEGFR2. These results suggest that SHP-1, not SHP-2, is likely to be the key regulator of VEGFR2 dephosphorylation in response to TSR and are consistent with previous reports that VEGFR2 Tyr1175 is dephosphorylated by SHP-1 but not SHP-2.
To validate the biochemical studies described here we also performed 2-color confocal immunofluorescence microscopy on MVEC treated with and without VEGF/TSR. Cells incubated with murine monoclonal anti–SHP-1 and rabbit anti-CD36 were imaged after incubating with Alexa Fluor 488– and Alexa Fluor 594–conjugated second antibodies. As shown in Figure 1D, under “resting” conditions or after exposure to VEGF alone, the 2 antibodies had mostly nonoverlapping fluorescence patterns. In the presence of TSR, however, there was significant colocalization of the antibodies in a peripheral surface pattern, suggesting that SHP-1 accumulates at membrane-associated sites containing CD36. This pattern was accentuated when cells were incubated with both TSR and VEGF. These results are consistent with a model wherein TSR induces association of active SHP-1 with VEGFR2, resulting in tyrosine dephosphorylation of the receptor upon VEGF treatment.
SHP-1 mediates TSR-induced dephosphorylation of pVEGFR2
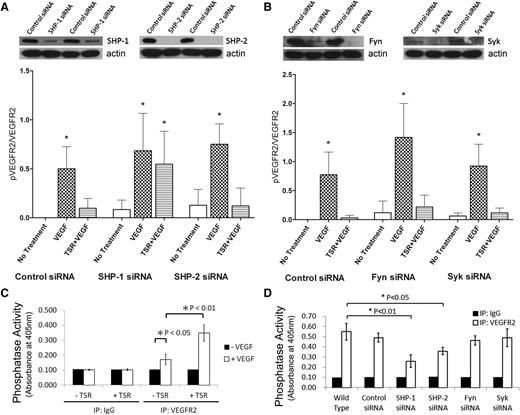
To determine the role of SHP phosphatases in the down-modulation of VEGFR2 phosphorylation by TSR, we used specific siRNAs to silence SHP-1 and SHP-2 expression in MVEC. Expression levels of SHP-1 and SHP-2 were reduced by an average of 64% and 98% by the siRNAs, respectively (Figure 2A, top). Because an antibody against phospho–SHP-1 was not available, we assessed the total phosphatase activity of anti-VEGFR2 immunoprecipitates in vitro. As is shown in Figure 2C, in the absence of VEGF, no phosphatase activity was detected. VEGF alone induced a modest increase in phosphatase activity (P < .05), consistent with previous reports,21,23 but pretreatment with TSR increased VEGF-induced phosphatase activity within the VEGFR2 complex more than twofold (P < .01). Although silencing either SHP-1 or SHP-2 significantly (P < .01) decreased the phosphatase activity associated with the VEGFR2 complex after VEGF and TSR treatment (Figure 2D), only silencing SHP-1 abrogated TSR-induced reduction in VEGF-dependent VEGFR2 phosphorylation (Figure 2A, bottom). These data are consistent with the observations that SHP-2 is constitutively associated with VEGFR2 and activated by VEGF, but not required for dephosphorylation of VEGFR2 Tyr1175 by TSR. Interestingly, silencing Fyn and Syk, nonreceptor tyrosine kinases that are known to be involved in the CD36/VEGFR2 pathway, had no effect on the phosphatase activity within the VEGFR2 complex, VEGF-induced VEGFR2 Tyr1175 phosphorylation, or VEGFR2 dephosphorylation by TSR (Figure 2B). Mean reductions in the expression of Fyn and Syk by the siRNAs were 53% and 85%, respectively (Figure 2B, top).
Inhibition of VEGF-induced VEGFR2 phosphorylation by TSR is mediated through SHP-1. MVEC transfected with control siRNA or siRNA directed against SHP-1 or SHP-2 (A) or against Fyn or Syk (B) were exposed to 10 nmol/L TSR in low-serum medium for 4 hours and then to 50 ng/mL VEGF for 5 minutes. Phosphorylated VEGFR2 in these samples were analyzed by western blot with pTyr1175 antibody. Blots were reprobed for VEGFR2 and phosphorylation levels expressed as a ratio of phosphorylated to total VEGFR2 (n = 3). The images above the bar graphs are representative western blots showing the efficiency of protein knock-down 48 hours after transfection. Anti-VEGFR2 or anti-IgG control immunoprecipitates of MVEC (C) or MVEC silenced with respective siRNA (D) were incubated with the phosphatase substrate pNPP for 1 hour at room temperature, and phosphatase activity was detected by absorbance at 405 nm.
Inhibition of VEGF-induced VEGFR2 phosphorylation by TSR is mediated through SHP-1. MVEC transfected with control siRNA or siRNA directed against SHP-1 or SHP-2 (A) or against Fyn or Syk (B) were exposed to 10 nmol/L TSR in low-serum medium for 4 hours and then to 50 ng/mL VEGF for 5 minutes. Phosphorylated VEGFR2 in these samples were analyzed by western blot with pTyr1175 antibody. Blots were reprobed for VEGFR2 and phosphorylation levels expressed as a ratio of phosphorylated to total VEGFR2 (n = 3). The images above the bar graphs are representative western blots showing the efficiency of protein knock-down 48 hours after transfection. Anti-VEGFR2 or anti-IgG control immunoprecipitates of MVEC (C) or MVEC silenced with respective siRNA (D) were incubated with the phosphatase substrate pNPP for 1 hour at room temperature, and phosphatase activity was detected by absorbance at 405 nm.
CD36 is required for TSR-induced SHP-1 association with VEGFR2 and VEGFR2 dephosphorylation in MVEC
To test whether the association of SHP-1 with VEGFR2 is mediated by CD36, siRNA was used to silence CD36 expression in cultured MVEC. As shown in Figure 3A, CD36 expression was dramatically decreased 48 hours after siRNA transfection, with no effect seen with the scrambled siRNA control. Although TSR dramatically inhibited VEGF-induced VEGFR2 phosphorylation in cells transfected with the control siRNA, the inhibitory effect was completely lost in CD36-silenced cells (Figure 3B), demonstrating that CD36 is required for TSR-mediated VEGFR2 dephosphorylation. As an additional control, we showed that human umbilical vein endothelial cells, which do not express CD36, showed no inhibitory effect of TSR (supplemental Figure 3). As shown by immunoprecipitation in Figure 3C (both panels), TSR-induced SHP-1 association with VEGFR2 (lane 2) was not seen in cells transfected with CD36 siRNA (lane 4).
CD36 is required for TSR-induced SHP-1 association with VEGFR2 and VEGFR2 dephosphorylation in MVEC. (A) MVEC were transfected with CD36 or control siRNA for 6 hours and then cultured for 48 hours before analysis of CD36 expression by immunoprecipitation and western blot. (B) MVEC transfected as in (A) were treated with 10 nmol/L TSR in low-serum medium for 4 hours and then exposed to 50 ng/mL VEGF for 5 minutes. VEGFR2 phosphorylation was analyzed by western blot with an antibody to pTyr1175. Blots were reprobed with an antibody to VEGFR2. The level of phosphorylation was shown as the ratio of phosphorylated to total VEGFR2 (B). (C) MVEC transfected as in (B) were treated with 10 nmol/L TSR in low-serum medium for 4 hours and then either exposed (left) or not exposed (right) to 50 ng/mL VEGF for 15 minutes. VEGFR2 was then immunoprecipitated and the precipitates were analyzed by western blot with anti–SHP-1 antibody. Blots were reprobed with an antibody to VEGFR2. Blots are representative of 3 experiments.
CD36 is required for TSR-induced SHP-1 association with VEGFR2 and VEGFR2 dephosphorylation in MVEC. (A) MVEC were transfected with CD36 or control siRNA for 6 hours and then cultured for 48 hours before analysis of CD36 expression by immunoprecipitation and western blot. (B) MVEC transfected as in (A) were treated with 10 nmol/L TSR in low-serum medium for 4 hours and then exposed to 50 ng/mL VEGF for 5 minutes. VEGFR2 phosphorylation was analyzed by western blot with an antibody to pTyr1175. Blots were reprobed with an antibody to VEGFR2. The level of phosphorylation was shown as the ratio of phosphorylated to total VEGFR2 (B). (C) MVEC transfected as in (B) were treated with 10 nmol/L TSR in low-serum medium for 4 hours and then either exposed (left) or not exposed (right) to 50 ng/mL VEGF for 15 minutes. VEGFR2 was then immunoprecipitated and the precipitates were analyzed by western blot with anti–SHP-1 antibody. Blots were reprobed with an antibody to VEGFR2. Blots are representative of 3 experiments.
CD36 and SHP-1 are required for TSR inhibition of MVEC migration and tube formation
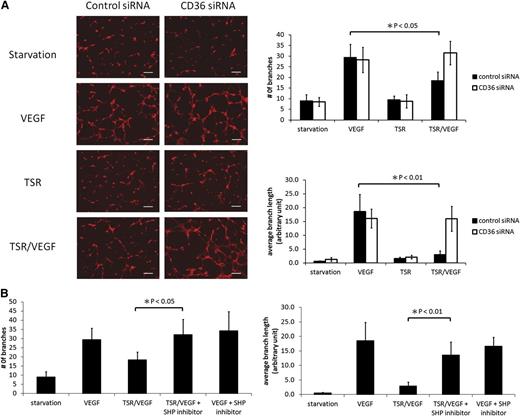
To understand the functional significance of CD36 and SHP-1 in the TSR-VEGFR2 signaling pathway, we assessed MVEC migration and tube formation in vitro. After transfecting MVEC with specific siRNA species, Transwell migration assays were performed and migrated cells quantified as a ratio of VEGF-treated cells to those not treated with VEGF. As is shown in Figure 4A, TSR inhibited VEGF-induced MVEC migration (P < .01) in control siRNA-transfected cells, and the inhibition was significantly reversed (P < .01) by transfection with SHP-1 siRNA, but not by SHP-2 siRNA or control-scrambled siRNA. Also, although MVEC rarely formed tubelike structures when cultured on Matrigel in low-serum medium, exposure to VEGF significantly induced tube formation (Figure 5A). Pretreatment with TSR inhibited tube formation as assessed by both the number of branches (P < .05) and the average branch length (P < .01) of tubelike structures in cells transfected with control siRNA, but not in CD36 siRNA-transfected cells. To elucidate the role of SHP-1 in this inhibition, MVEC were treated with TSR and then exposed to VEGF in the presence of NSC-87877 (Figure 5B). The SHP-1 inhibitor significantly rescued tube formation in the TSR-treated cell cultures as assessed by both branch number (P < .05) and mean branch length (P < .01). To exclude the possibility that the SHP inhibitor itself induced tube formation, cells were treated with VEGF plus the inhibitor without TSR, and no significant changes were seen. These results show that preventing TSR-mediated VEGFR2 dephosphorylation by silencing or inhibiting CD36 or SHP-1 has functional consequences on MVEC angiogenic behavior.
SHP-1 is required for TSR-mediated inhibition of VEGF-induced MVEC migration. (A) MVEC transfected with specific siRNA as in Figure 2 were allowed to migrate toward VEGF for 15 hours through an 8.0-μm membrane in a Transwell insert in the presence or absence of 10 nmol/L TSR. Within each group, siRNA transfected MVEC not exposed to VEGF or TSR served as controls. The number of migrated cells was counted and the result expressed as percent of cells migrated compared with control (n = 8). (B) Migration assays as in panel A were performed with MVEC treated with Src inhibitor (PP2) or Syk inhibitor (Piceatannol) (n = 6). PP3 served as a negative control for PP2.
SHP-1 is required for TSR-mediated inhibition of VEGF-induced MVEC migration. (A) MVEC transfected with specific siRNA as in Figure 2 were allowed to migrate toward VEGF for 15 hours through an 8.0-μm membrane in a Transwell insert in the presence or absence of 10 nmol/L TSR. Within each group, siRNA transfected MVEC not exposed to VEGF or TSR served as controls. The number of migrated cells was counted and the result expressed as percent of cells migrated compared with control (n = 8). (B) Migration assays as in panel A were performed with MVEC treated with Src inhibitor (PP2) or Syk inhibitor (Piceatannol) (n = 6). PP3 served as a negative control for PP2.
CD36 and SHP-1 are required for TSR inhibition of MVEC tubelike structure formation. (A) MVEC were transfected with CD36 or control siRNA as in Figure 3 and then treated with 10 nmol/L TSR in low-serum medium for 4 hours before being transferred onto Matrigel-coated tissue culture wells. Cells were then exposed to 50 ng/mL VEGF for 6 hours, fixed with 4% paraformaldehyde, and then stained with 30 nmol/L fluorescence-labeled Phalloidin for 1 hour. Images from 4 randomly chosen areas were obtained with a fluorescence microscope, and the number of branches and average branch length of tubelike structures were quantified using NIH ImageJ software. The scale bar represents 200 μm. (B) MVEC were transfected with control siRNA and treated with TSR as in panel A. Cells were then exposed to 50 ng/mL VEGF for 6 hours in the presence of 100 μmol/L SHP inhibitor NSC-87877 or vehicle control. The number of branches and the average branch length of tubelike structures were analyzed as in panel A.
CD36 and SHP-1 are required for TSR inhibition of MVEC tubelike structure formation. (A) MVEC were transfected with CD36 or control siRNA as in Figure 3 and then treated with 10 nmol/L TSR in low-serum medium for 4 hours before being transferred onto Matrigel-coated tissue culture wells. Cells were then exposed to 50 ng/mL VEGF for 6 hours, fixed with 4% paraformaldehyde, and then stained with 30 nmol/L fluorescence-labeled Phalloidin for 1 hour. Images from 4 randomly chosen areas were obtained with a fluorescence microscope, and the number of branches and average branch length of tubelike structures were quantified using NIH ImageJ software. The scale bar represents 200 μm. (B) MVEC were transfected with control siRNA and treated with TSR as in panel A. Cells were then exposed to 50 ng/mL VEGF for 6 hours in the presence of 100 μmol/L SHP inhibitor NSC-87877 or vehicle control. The number of branches and the average branch length of tubelike structures were analyzed as in panel A.
Because the nonreceptor tyrosine kinases Fyn and Syk are involved in CD36/VEGFR2 signaling pathways, we determined the impact of their silencing by siRNA on MVEC migration. As shown in Figure 4A, silencing Fyn did not significantly reverse TSR inhibition of migration in the Boyden chamber assay, consistent with the lack of effect on VEGFR2 phosphorylation (Figure 2). Interestingly, Syk silencing significantly reversed (P < .01) TSR-inhibited cell migration (Figure 4A) even though it did not affect VEGFR2 phosphorylation. To validate this finding, we also applied a pharmacologic approach. Broad-spectrum pharmacologic inhibition of Src-family kinases with PP2 had no effect on TSR-mediated inhibition of migration, whereas a specific chemical inhibitor of Syk kinase (Piceatannol) significantly reversed the TSR effect (Figure 4B; P < .01).
Cd36 null mice exhibit increased VEGFR2 phosphorylation and decreased SHP-1 association with VEGFR2 after VEGF infusion
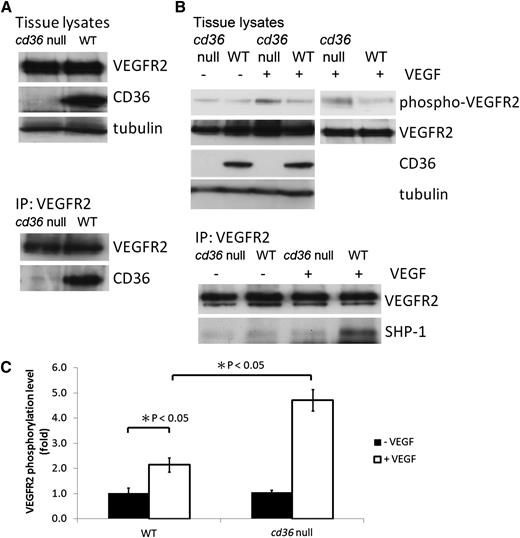
To elucidate the role of CD36 in the inhibition of VEGF signaling in vivo, we assayed VEGFR2 phosphorylation in the lung tissue of cd36 null mice treated with an intravenous infusion of VEGF. Endothelial cells are the most predominant cells in lung tissue. As shown by western blot analysis in Figure 6A, CD36 and VEGFR2 were expressed in the lung tissue of wild-type mice, and deletion of cd36 had no effect on the level of expression of VEGFR2. As with cultured MVEC, CD36 was coprecipitated from wild-type lung tissue by anti-VEGFR2, suggesting that CD36 and VEGFR2 form a complex in vivo. Lack of coprecipitation from cd36 null lung tissues demonstrated specificity. To induce VEGFR2 phosphorylation in vivo, 1 μg of VEGF was injected via the jugular vein 5 minutes before harvesting the lung tissue. As is shown in Figure 6B-C, VEGF induced a twofold increase in the level of phosphorylation of VEGFR2 in tissue from wild-type mice compared with the basal level seen in nontreated mice (P < .05). Lung tissue from cd36 null mice, however, showed a 4.7-fold increase in VEGFR2 phosphorylation after VEGF infusion (P < .05 comparing wild-type with cd36 null), suggesting that endogenous CD36 signaling suppressed VEGF-induced VEGFR2 phosphorylation in vivo. Anti-VEGFR2 immunoprecipitates from these tissues revealed substantially less SHP-1 associated with VEGFR2 after VEGF injection in cd36 null mice compared with wild-type, suggesting that CD36 is required for VEGF-mediated recruitment of SHP-1 to the VEGFR2 complex. We verified by western blot analysis of lung tissues that there was no significant difference in SHP-1 expression in cd36 null mice compared with wild-type (data not shown).
Genetic deletion of cd36 increases VEGFR2 phosphorylation and decreases SHP-1 association with VEGFR2 after in vivo infusion of VEGF. (A) Lysates of lung tissue removed from cd36 null or wild-type mice were analyzed by western blot with anti-VEGFR2, anti-CD36, or antitubulin (top) or by immunoprecipitation with anti-VEGFR2 followed by western blot (bottom). (B) Lung tissue from wild-type or cd36 null mice was harvested 5 minutes after injection of 1 μg VEGF via the jugular vein. The top panel shows VEGFR2 Tyr1175 phosphorylation detected by western blot analysis of tissue lysates. Blots were reprobed with antibodies to total VEGFR2, CD36, and tubulin. The bottom panel shows western blot analysis of anti-VEGFR2 immunoprecipitates of the tissue lysates probed with antibodies to SHP-1 and VEGFR2. Blots are representative of 3 experiments. (C) VEGFR2 phosphorylation levels in panel B were expressed as the ratio of phosphorylated VEGFR2 to total VEGFR2.
Genetic deletion of cd36 increases VEGFR2 phosphorylation and decreases SHP-1 association with VEGFR2 after in vivo infusion of VEGF. (A) Lysates of lung tissue removed from cd36 null or wild-type mice were analyzed by western blot with anti-VEGFR2, anti-CD36, or antitubulin (top) or by immunoprecipitation with anti-VEGFR2 followed by western blot (bottom). (B) Lung tissue from wild-type or cd36 null mice was harvested 5 minutes after injection of 1 μg VEGF via the jugular vein. The top panel shows VEGFR2 Tyr1175 phosphorylation detected by western blot analysis of tissue lysates. Blots were reprobed with antibodies to total VEGFR2, CD36, and tubulin. The bottom panel shows western blot analysis of anti-VEGFR2 immunoprecipitates of the tissue lysates probed with antibodies to SHP-1 and VEGFR2. Blots are representative of 3 experiments. (C) VEGFR2 phosphorylation levels in panel B were expressed as the ratio of phosphorylated VEGFR2 to total VEGFR2.
Discussion
TSP-1 was identified as an angiogenesis inhibitor more than 20 years ago.26,27 The mechanisms underlying this activity were initially demonstrated to involve a specific MVEC receptor, CD36, which generates antiangiogenic signals that lead to apoptosis in the presence of angiogenic growth factors.4,8 An inhibitory effect of TSP-1 on VEGF-induced VEGFR2 phosphorylation is a more recent observation,10 although molecular mechanisms have not been identified. In this manuscript we defined 2 mechanistic components of the pathway. We used CD36 siRNA-transfected MVEC and cd36 null mice to provide evidence that CD36 is required for TSR-mediated VEGFR2 dephosphorylation in vitro and in vivo. We also found that the suppression of VEGF-induced VEGFR2 phosphorylation by TSP-1 or TSR peptide was mediated by CD36-dependent recruitment of a specific phosphatase, SHP-1, to the VEGFR2 signaling complex.
CD36 was previously shown by Zhang et al to associate with VEGFR2 in cultured MVEC.10 Similar to what was reported by this group, we also found that detection of the CD36-VEGFR2 interaction depended on the choice of detergent used to lyse the cells (data not shown), suggesting perhaps that the interaction occurs in specific membrane microdomains.28,29 Here we report for the first time that the association of CD36 with VEGFR2 in vivo in murine lung has functional consequences with regard to VEGF response. Our in vivo finding that VEGF-induced VEGFR2 phosphorylation was enhanced by genetic deletion of cd36 suggests that physiological levels of TSP-1 or other endogenous CD36 ligands normally dampen VEGF signaling. The concentration of TSP-1 and TSR required to dephosphorylate VEGFR2 phosphorylated by VEGF in MVEC is at the nanomolar level, well within the range of reported plasma concentrations of TSP-1.30 Our results are also consistent with the observation that endogenous levels of TSP-1 were sufficient to inhibit VEGF-induced Akt phosphorylation in the mouse retina.11
SHP-1 was reported to associate with VEGFR2 constitutively in human umbilical vein endothelial cells,21 whereas in VEGFR2-transfected porcine aortic endothelial cells, the association required induction by VEGF.31 In murine lung in vivo we now show that the association was CD36-dependent and induced by VEGF. In vitro in human MVEC, however, the association was neither constitutive nor induced by VEGF, but rather it was induced by TSR in a CD36-dependent manner. Unlike in vitro, endothelial cells in vivo are constantly exposed to low levels of VEGF and other TSR-containing proteins that could account for these differences. The association of SHP-1 with target proteins is mediated by the binding of its SH2 domain to phospho-tyrosine residues. It is possible that the multiple potential sites for tyrosine phosphorylation on VEGFR2 that can be SHP-1 targets are differentially phosphorylated under basal conditions in endothelial cells from different sites so that some cells require VEGF stimulation to create a docking site, whereas others constitutively present a docking site. CD36 is not known to contain any phospho-tyrosine, but it does form a signaling complex with adaptor proteins, such as Vav, that could function in this regard.32
Although other studies have shown by using siRNA that SHP-1 is the critical phosphatase that regulates VEGFR2 in endothelial cells,20,21 we now show with an in vitro assay that phosphatase activity within the VEGFR2 complex was induced by VEGF and enhanced by TSR in a CD36-dependent manner. Although this is consistent with our data showing that TSR-induced association of SHP-1 with VEGFR2, we further demonstrated using siRNA that SHP-1 was required for TSR-mediated VEGFR2 dephosphorylation and contributed to the phosphatase activity within the VEGFR2 complex. The physiological relevance of these findings was shown with in vitro endothelial tube formation and migration assays, in which we demonstrated using both pharmacologic and genetic approaches that inhibition of VEGF signaling by TSR was mediated by CD36 and SHP-1. Although the SHP inhibitor blocks both SHP-1 and SHP-2 activity,33,34 our data show that the association of SHP-2 was not affected by VEGF or TSR, and that SHP-2 did not contribute to TSR-induced VEGFR2 dephosphorylation or inhibition of migration in MVEC. Our work demonstrates, in vitro and in vivo, that CD36, SHP-1, and VEGFR2 are components of a ligand-dependent, detergent-sensitive, multiprotein complex. The precise molecular mechanisms mediating these protein-protein interactions remain to be determined, although previous studies show that the carboxy-terminal cytoplasmic tail of CD36 was sufficient to precipitate a signaling complex from macrophages.35 Because Fyn kinase is known to function downstream of CD36 in MVEC proapoptotic responses to TSR, we also examined the role of this kinase in TSR regulation of VEGFR2 phosphorylation. Genetic and pharmacologic approaches showed that Fyn was not required, suggesting that the TSR/CD36-mediated proapoptotic signaling pathway differs from the SHP-1–mediated pathway that regulates VEGFR2 phosphorylation and MVEC migration and tube formation.
We also probed the role of Syk kinase in the TSR/CD36 pathway because it has been reported to phosphorylate VEGFR2 on Tyr1175 and it may function downstream of CD36.36 Interestingly, we found by using both genetic and pharmacologic approaches that inhibition of Syk abrogated TSR/CD36-mediated inhibition of VEGF-induced MVEC migration but had no effect on VEGFR2 Tyr1175 phosphorylation. The latter result is different from that reported by Kazerounian et al36 and is likely related to the use of a different inhibitor or maybe a different MVEC system. The discordance between inhibition of the TSR effect on migration and lack of TSR effect on VEGFR2 dephosphorylation could be related to the different kinetics of the assays. The phosphorylation studies assessed an acute (5 minutes) response to VEGF stimulation and are consistent with the abundant data showing that VEGFR2 Tyr1175 is auto-phosphorylated upon VEGF stimulation,37,38 whereas the migration assays were carried out over many hours. We thus hypothesize that Syk activation by CD36 contributes to phosphorylation and activation of SHP-1 in the long-term inhibition of VEGF-induced cell migration by TSR. This model is consistent with known functions of the Syk homolog, ZAP70, which has been shown to interact with SHP-1 and enhance its phosphatase activity via phosphorylation of Tyr536 and Tyr564.39-42
Previous work from our laboratory demonstrated that CD36 signaling in macrophages in response to oxidized phospholipids results in downregulation of SHP-2 activity.24 Mechanistically this was because of generation of intracellular oxidant stress and oxidative modification of a critical cysteine residue at the active site of the phosphatase. Endothelial cells signaling by TSR is fundamentally different because SHP-2 was not affected, yet SHP-1 activity was increased. This difference could be a result of the use of different CD36 ligands (TSR peptide vs oxidized phospholipid) and/or differences in downstream Src-family or mitogen-activated protein kinases in the different cell types.
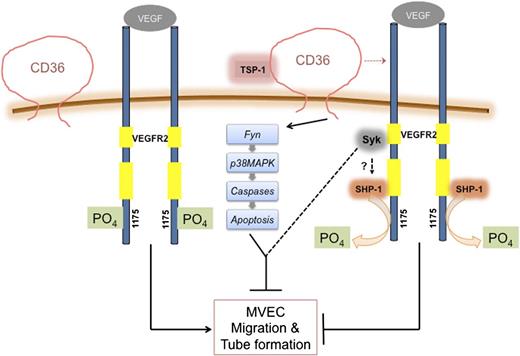
In summary, we showed that binding of TSP-1 or TSR to CD36 suppressed VEGF-induced VEGFR2 phosphorylation via CD36-dependent recruitment of SHP-1 phosphatase to the VEGFR2 signaling complex (Figure 7). These studies identify a novel mechanistic regulatory pathway that could potentially be targeted to enhance or inhibit angiogenesis in a therapeutic context.
Model depicting CD36-dependent antiangiogenic pathways. VEGFR2 forms a complex with CD36. When CD36 is not engaged by its ligand, TSP-1, VEGF binding to VEGFR2 induces VEGFR2 phosphorylation at Tyr1175 and promotes MVEC migration and tube formation. Engagement of CD36 by TSP-1 leads to inhibition of migration and tube formation by 2 independent processes. Recruitment of SHP-1 to the VEGFR2-CD36 complex leads to dephosphorylation of VEGFR2 (Tyr1175), thus dampening VEGF signaling, and activation of Fyn leads to apoptosis via activation of MAP kinases and caspases. Syk may participate in these pathways, although the mechanisms remain undefined.
Model depicting CD36-dependent antiangiogenic pathways. VEGFR2 forms a complex with CD36. When CD36 is not engaged by its ligand, TSP-1, VEGF binding to VEGFR2 induces VEGFR2 phosphorylation at Tyr1175 and promotes MVEC migration and tube formation. Engagement of CD36 by TSP-1 leads to inhibition of migration and tube formation by 2 independent processes. Recruitment of SHP-1 to the VEGFR2-CD36 complex leads to dephosphorylation of VEGFR2 (Tyr1175), thus dampening VEGF signaling, and activation of Fyn leads to apoptosis via activation of MAP kinases and caspases. Syk may participate in these pathways, although the mechanisms remain undefined.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Bin Ren (Medical College of Wisconsin) for his helpful suggestions.
This work was supported by National Institutes of Health grant HL085718 (R.L.S).
L.-Y.C. was, and D.P.R. is, a PhD candidate at Case Western Reserve University, and this work is submitted in partial fulfillment of their thesis requirement.
Authorship
Contribution: L.-Y.C. and D.P.R. designed, performed, and analyzed the results and wrote the manuscript; and R.L.S contributed to the overall project design and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of L.-Y.C. is the Institute of Cellular and System Medicine, National Health Research Institutes, Miaoli, Taiwan.
Correspondence: Roy L. Silverstein, Chair, Department of Medicine, Medical College of Wisconsin, 9200 W Wisconsin Ave., C5038, Milwaukee, WI 53226; e-mail: rsilverstein@mcw.edu.
References
Author notes
L.-Y.C. and D.P.R. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal