In this issue of Blood, Abel et al designed lentiviral vectors (LVs) enabling specific gene delivery into endothelial cells in vivo. This opens new perspectives for gene therapy of hereditary disorders, cardiovascular diseases, and cancer.1
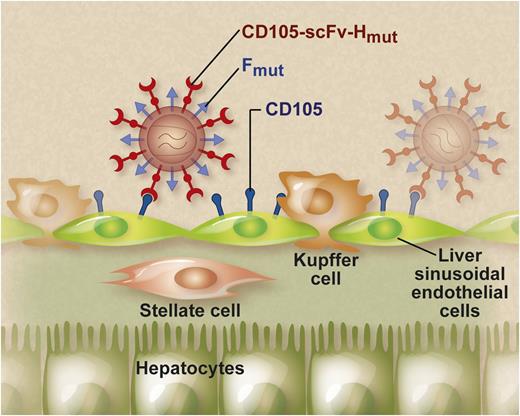
LVs displaying endothelial-specific envelopes specifically target LSECs after systemic injection. This endothelial specificity was achieved using a chimeric measles virus envelope (CD105-scFv-Hmut) composed of an scFv specific for CD105 genetically linked to a mutated measles virus hemagglutinin H protein. The pseudotyped LV particles also displayed a mutated fusogenic measles virus F protein on their surface to mediate entry into the target cells. The exquisite endothelial specificity of these CD105-specific LVs was confirmed in tumor-derived endothelium and in human veins and arteries ex vivo. Professional illustration by Debra T. Dartez.
LVs displaying endothelial-specific envelopes specifically target LSECs after systemic injection. This endothelial specificity was achieved using a chimeric measles virus envelope (CD105-scFv-Hmut) composed of an scFv specific for CD105 genetically linked to a mutated measles virus hemagglutinin H protein. The pseudotyped LV particles also displayed a mutated fusogenic measles virus F protein on their surface to mediate entry into the target cells. The exquisite endothelial specificity of these CD105-specific LVs was confirmed in tumor-derived endothelium and in human veins and arteries ex vivo. Professional illustration by Debra T. Dartez.
The recent clinical successes in gene therapy are fueling renewed interest in the field. Sustained therapeutic benefits have been repeatedly observed in patients with a wide variety of severe genetic disorders and cancer.2 These advances mirror the continuous improvements in gene delivery technologies. Nevertheless, there is still a need to develop vectors that can specifically deliver their therapeutic gene cargo into the desired target cells while preventing unwarranted genetic modification of nontarget cells. One of the “holy grails” in gene therapy focuses on the identification of robust cell-specific “targetable” vectors that can accomplish such a feat. The specific delivery of genes into endothelial cells has been particularly challenging.3 In this issue, Abel et al have now overcome this bottleneck and demonstrate, for the first time, targeted gene delivery to endothelial cells on systemic LV injection.1
LVs are attractive vehicles for in vivo gene therapy. These vectors integrate their therapeutic cargo stably into the target cell genome, enabling its sustained expression.4 LVs are surrounded by a lipid bilayer studded with envelope proteins. These envelope proteins bind to their cognate cellular receptors and consequently determine LV tropism. Typically, LVs are “pseudotyped” with envelope proteins derived from other enveloped viruses different from the parental HIV-1 virus from which they are derived.4 In particular, it is possible to embed envelope proteins into LVs that display cell type-specific targeting moieties.5 In this issue, Abel et al demonstrated that endothelial-specific gene transfer in vivo could be achieved using LVs that are pseudotyped with endothelial-specific envelopes.5 This endothelial specificity was achieved using chimeric measles virus envelopes (designated as CD105-scFv-Hmut) composed of a single-chain variable antibody fragment (scFv) specific for the endothelial-specific marker endoglin (CD105) genetically linked to a mutated measles virus hemagglutinin H protein that does not bind its natural receptor, as described previously (see figure).5 The pseudotyped LV particles also displayed a mutated fusogenic measles virus F protein on their surface to mediate entry into the target cells. The cytoplasmic tails of the H and F envelopes were truncated to increase vector titer. Systemic injection of these CD105-targeted LV in mice resulted in selective gene transfer into liver sinusoidal endothelial cells (LSECs). In contrast, hepatocytes, Kupffer cells, and stellate cells were relatively refractory to the CD105-targeted LVs (see figure). Similarly, LVs specifically targeted to human CD105 transduced exclusively human LSECs after xenotransplantation in mice. These properties distinguish CD105-targeted LVs from conventional LVs pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G). VSV-G pseudotyping allows for stable in vivo gene transfer in different tissues, but their lack of specificity can potentially cause unwanted adverse effects. In particular, VSV-G–pseudotyped LVs transduce not only hepatocytes and LSECs but also antigen-presenting cells in the liver (ie, Kupffer cells) and spleen.6 Ectopic expression of the therapeutic transgene in these antigen-presenting cells may, in turn, provoke an untoward immune reaction that curtails long-term gene expression of the therapeutic gene product.7,8 The use of CD105-targeted LVs may potentially overcome this potential limitation. In addition, the location and natural function of LSECs make them particularly well suited for the targeted release of therapeutic proteins into the blood stream after gene therapy with these CD105-targeted LVs, which has important clinical implications. In particular, Abel et al demonstrated that the CD105-targeted LVs could be used to express therapeutically relevant secreted proteins, such as erythropoietin, that consequently resulted in a significant and sustained increase in hematocrit levels. Because endothelial cells likely represent the main natural cell source of coagulation factor VIII, this LSEC-targeted approach has implications for hemophilia A gene therapy.9 Moreover, LSECs are believed to play a role in induction of immune tolerance.10 These properties may be essential to reduce the risk for the development of immune responses against the therapeutic protein and/or the gene modified cells. Nevertheless, factor VIII expression is more challenging and will likely require more robust expression cassettes and higher vector doses. Additional studies are warranted to address these outstanding questions.
The endothelial specificity of these CD105-targeted LVs is not restricted to LSECs. The human CD105-targeted LVs were also able to transduce endothelial cells of larger blood vessels, such as human saphenous veins and human arteries ex vivo. Similarly, the human CD105-targeted LVs also transduced human arteries in a xenotransplantation model. In addition, intratumoral delivery of CD105-targeted LVs resulted in selective gene delivery into the tumor-derived endothelium in the absence of gene transfer into LSECs. Endothelial cells in tumor tissue were also transduced on systemic administration of the CD105-targeted LV, although this required higher vector doses and also resulted in transduction of LSECs. This finding has possible implications for the treatment of metastatic lesions, provided the efficiency and specificity for tumor endothelium can be further increased.
The CD105 marker is predominantly expressed on cells of the microvascular endothelium (eg, in the liver) and on endothelial cells during active angiogenesis, as in the case of tumors. This expression pattern is consistent with the tropism of the CD105-targeted LVs and suggests that high CD105 expression is necessary to enable efficient transduction. Nevertheless, vector biodistribution also depends on the accessibility of the target cells and hemodynamic parameters. This pattern may explain the predominant transduction of LSECs after systemic vector administration. The lower levels of CD105 expression and/or the relative inaccessibility of hepatic stellate cells in the space of Disse may account for their poor transduction. One potential caveat of the CD105-targeted LVs is their relatively low titer compared with conventional VSV-G–pseudotyped LVs. Moreover, the prevalence in the human population of antibodies to the measles virus hemagglutinins may ultimately block in vivo gene transfer with these CD105-targeted LVs. These issues would need to be addressed.
Because endothelial cells play an important role in various physiological and pathological processes, this technological advance opens new perspectives for treatment of genetic and cardiovascular disease and cancer. In addition, the CD105-targeted LV represents a useful technology for fundamental research in endothelial cell biology. As we gain a better understanding in the molecular signatures that define distinct endothelial cell populations, future studies may exploit these insights to fine-tune transduction into specific endothelial cell subsets.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal