Key Points
The development and survival of mature NKT cells are impaired in DOCK8-deficient mice.
DOCK8 is required for antigen-induced NKT cell proliferation and cytokine production.
Abstract
Patients with the dedicator of cytokinesis 8 (DOCK8) immunodeficiency syndrome suffer from recurrent viral and bacterial infections, hyper–immunoglobulin E levels, eczema, and greater susceptibility to cancer. Because natural killer T (NKT) cells have been implicated in these diseases, we asked if these cells were affected by DOCK8 deficiency. Using a mouse model, we found that DOCK8 deficiency resulted in impaired NKT cell development, principally affecting the formation and survival of long-lived, differentiated NKT cells. In the thymus, DOCK8-deficient mice lack a terminally differentiated subset of NK1.1+ NKT cells expressing the integrin CD103, whereas in the liver, DOCK8-deficient NKT cells express reduced levels of the prosurvival factor B-cell lymphoma 2 and the integrin lymphocyte function-associated antigen 1. Although the initial NKT cell response to antigen is intact in the absence of DOCK8, their ongoing proliferative and cytokine responses are impaired. Importantly, a similar defect in NKT cell numbers was detected in DOCK8-deficient humans, highlighting the relevance of the mouse model. In conclusion, our data demonstrate that DOCK8 is required for the development and survival of mature NKT cells, consistent with the idea that DOCK8 mediates survival signals within a specialized niche. Accordingly, impaired NKT cell numbers and function are likely to contribute to the susceptibility of DOCK8-deficient patients to recurrent infections and malignant disease.
Introduction
Natural killer T (NKT) cells are a rare population of immunoregulatory T lymphocytes that influence a broad range of diseases including infection, cancer, autoimmunity, and allergy.1-5 The main subset of these cells express a semi-invariant T-cell receptor (TCR) composed in mice of a Vα14-Jα18 rearrangement, which preferentially associates with the Vβ8, Vβ7, or Vβ2 TCR β chains. These TCRs bind to lipid-based antigens presented by the nonclassical major histocompatibility complex molecule CD1d.6 Although it is increasingly clear that there are many different physiologically-relevant antigenic targets for NKT cells, the prototypic antigen recognized by these cells is α-galactosylceramide (αGalCer), a glycolipid originally isolated from a marine sponge (Agelis mauritianus). Through the use of this antigen, great progress has been made in understanding NKT cell development and function, including the ability to identify them in mice and humans using αGalCer-loaded CD1d tetramers.7
NKT cell development begins within the thymus before further development and maturation in the peripheral tissues. In the thymus, CD4+CD8+ double positive progenitor cells that randomly express the invariant TCR undergo positive selection mediated by CD1d, as well as homotypic interactions with signalling lymphocyte activation molecule (SLAM) family receptors, expressed on other double positive thymocytes.8,9 Selection of precursor CD24+NK1.1− NKT cells (stage 0) leads to a proliferative burst generating a large population of immature CD44−NK1.1− NKT cells (stage 1), which further develop to an intermediate stage of migratory CD44+NK1.1− NKT cells (stage 2) responsible for populating the peripheral tissues.8,9 The final maturation of these cells may occur in either the thymus or peripheral tissues. Maturation of cells within the thymus leads to the formation of a large, slowly dividing population of resident CD44+NK1.1+ NKT cells,10,11 whereas migration into the periphery under the influence of sphingosine-1-phosphate12 leads to the establishment of liver and splenic populations of fully mature NK1.1+ NKT cells.13,14
Dedicator of cytokinesis 8 (DOCK8) is a guanine nucleotide exchange factor (GEF) for Cdc4215 and belongs to the DOCK family of proteins, which are typically involved in the cytoskeletal rearrangements required to maintain cell structure, migration, integrin activation, lamellipodia formation, phagocytosis, and cell fusion.16,17 The importance of understanding the role of DOCK8 in host immunity has been underlined by the discovery of a rare primary immunodeficiency syndrome in individuals with loss-of-function mutations or deletions in DOCK8. DOCK8 immunodeficiency in humans leads to persistent and recurrent cutaneous infections with molluscum contagiosum, human papilloma virus, and herpes simplex virus, and recurrent sinopulmonary infections.18,19 Patients also have increased susceptibility to cancer, including metastatic squamous carcinoma18 and B-cell lymphoma.20 Given that NKT cells are important in immunity to infection and cancer and that NKT-cell development is dependent on the related DOCK2 protein,21 this raises the question of whether defects in NKT-cell development and/or function could contribute to an increased risk of infectious disease or malignancy in the absence of DOCK8.
Previous work in our laboratories has uncovered novel roles for DOCK8 in the generation of marginal zone (MZ) B cells, maintenance of germinal center B cells, and survival of memory T cells.22-24 However, a potential role of DOCK8 in NKT cell biology has yet to be defined. In this study, we show that humans and mice lacking DOCK8 have a peripheral deficiency in NKT cells, which is due to defects in survival, predominantly affecting fully mature, long-lived subsets. The findings show DOCK8-dependent signals are required to maintain these fully differentiated cells within their specialized environments and define a novel CD103+ subset of resident NKT cells in the thymus. DOCK8 deficiency results in diminished proliferative responses, specifically in these fully differentiated NKT cells, after TCR stimulation. The failure to sustain and activate NKT cells may therefore contribute to the increased susceptibility to infections and cancer in the DOCK8 immunodeficiency syndrome.
Material and methods
Human studies, mouse strains, and procedures
Human studies were approved by the institutional review board of the National Institute of Allergy and Infectious Diseases, the Sydney South West Area Health Service, and St. Vincent’s Hospital Darlinghurst Human Research Committees in accordance with Declaration of Helsinki principles. Animal experiments were approved by institutional ethical review committees and subject to home office license in the United Kingdom. DOCK8-deficient strains, Captain Morgan (CPM), DOCK8cpm/cpm and primuris (PRI) DOCK8pri/pri mice were generated by N-ethyl-N-nitrosourea mutagenesis.22 For bone marrow (BM) chimeras, C57BL/6 (CD45.1+) mice were irradiated with 2 fractions of 4.5 Gy and reconstituted with 5 × 106 to 10 × 106 BM cells for 8 to 10 weeks. C57BL/6-Tg(UBC-GFP)30Scha/J mice25 were crossed to DOCK8cpm/cpm mice to allow tracking of cells, and CD103 knockout [B6.129S2(C)-Itgaetm1Cmp/J] mice were purchased from The Jackson Laboratories.
Flow cytometry
Human peripheral blood mononuclear cells (PBMCs) and mouse lymphocytes were isolated and stained as previously described.18,22 PBMCs were stained with αGalCer-loaded tetramers or fluorescein isothiocyanate (FITC)-conjugated anti-human Vα24, phycoerythrin (PE)-conjugated anti-human Vβ11 (Beckman Coulter), and Pacific blue–conjugated anti-human CD3 (Biolegend) within 24 hours of collection. CD1d monomers were generated as previously described,26 loaded with αGalCer, and tetramerized using allophycocyanin-streptavidin. For murine lymphocytes, antibodies were from eBioscience: FITC-conjugated anti-CD24, BrdU, CD18, and CD103; PE-conjugated anti-αβTCR, interleukin-4 (IL-4), interferon γ (IFNγ), and CD11α; PE-Cy7–conjugated anti-CD69 and NK1.1; PerCP-Cy5.5–conjugated anti-B220 and CD8α; allophycocyanin-Cy7–conjugated anti-CD45.1, TCR, and CD44; and Pacific blue–conjugated anti CD44, B220, and CD45.1. QDot650 antibodies were against CD4, and live/dead kits (Aqua and Near-IR) from Invitrogen were used to distinguish live cells. Data were collected on FACSCanto or LSRII machines (Becton Dickinson) and analyzed with FlowJo (Tree Star, Inc.).
Adoptive transfers
Splenocytes from wild-type (WT) (CD45.2) green fluorescent protein (GFP), and DOCK8cpm/cpm GFP mice were enriched for NKT cells by negative selection using magnetic-activated cell sorting. WT (CD45.2)-enriched NKT cells were then mixed 1:1 with DOCK8cpm/cpm GFP or WT GFP NKT cells and transferred into CD45.1 recipients by intravenous injection. Lymphoid organs were harvested from recipients at designated time points, and ratios of adoptively transferred cells were analyzed.
Carboxyfluorescein diacetate succinimidyl ester proliferation assays
For in vitro proliferation assays, NK1.1+ and NK1.1– NKT cells were sorted from pooled thymi and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 0.5-1 μM, 8-10 minutes at room temperature or 37°C) before stimulation with anti-CD3/CD28 or αGalCer pulsed dendritic cells (sorted as CD11chi splenic cells). To carry out in vivo proliferation experiments, thymic NKT cells were labeled with CFSE before transfer into CD45.1 transgenic mice. After 24 hours, mice were injected with 1 μg αGalCer/mouse, and organs were harvested 4 days later.
RNA microarray experiments
The RNAqueous-Micro Kit (Ambion, Austin, TX) was used to isolate RNA samples as per the manufacturer’s protocols. Commercially available high-density oligonucleotide, MouseWG-6_V2 chips from Illumina (San Diego, CA), were used for whole-genome gene expression analysis. In brief, 500 ng of total RNA was reverse transcribed to synthesize first- and second-strand complementary DNA (cDNA), followed by in vitro transcription to synthesize biotin-labeled complementary RNA (cRNA) using the TotalPrep-96 RNA amplification kit from Ambion. A total of 1500 ng of biotin-labeled cRNA from each sample was used in the hybridization process at 58°C for 18 hours. The hybridized BeadChip was washed and labeled with streptavidin-Cy3 according to the manufacturer’s protocols. The accession number for the microarray data is GSE44816. Additionally, there are 2 individual subseries of data linked to the above accession number: GSE44814 and GSE44815.
Statistical data analysis
Statistical comparisons were performed using 2-tailed, unpaired t tests or Mann-Whitney tests. The significance of multiple comparisons was confirmed using Kruskal-Wallis tests where appropriate. More details are provided in the supplemental Methods on the Blood website.
Results
Deficiency in DOCK8 causes the loss of peripheral NKT cells
Investigation of the number of NKT cells in peripheral blood from patients with DOCK8 deficiency showed a marked reduction in αGalCer-tetramer+ NKT cells compared with healthy controls, often to undetectable levels (Figure 1A). Analysis of additional DOCK8-deficient patients using expression of CD3, Vα24, and Vβ11 to identify the NKT cells supported this finding (supplemental Figure 1). Because further phenotypic analysis of NKT cells from patients was limited due to difficulties in isolating NKT cells in sufficient numbers, to understand the significance of this observation, we turned to 2 N-ethyl-N-nitrosourea–derived DOCK8-deficient mouse strains. DOCK8cpm/cpm contains a homozygous null allele of DOCK8 due to a mutation in the splice donor site in exon 21 that truncates the native protein, whereas DOCK8pri/pri contains an inactivating mutation in the DHR2 (GEF) domain.22 DOCK8cpm/cpm and DOCK8pri/pri were studied in a parallel series of experiments, with no apparent differences between strains.
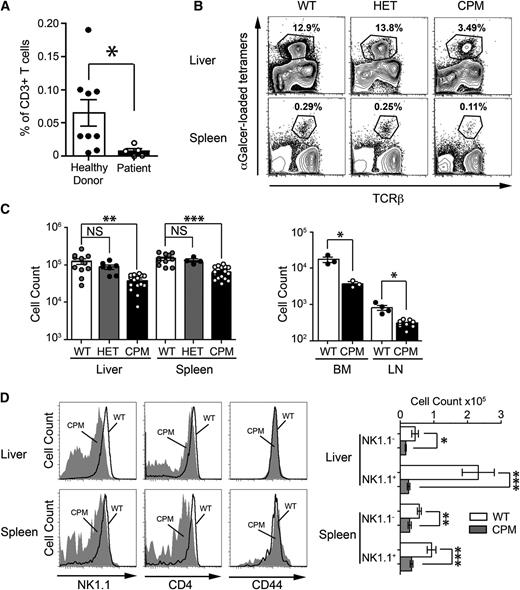
DOCK8 is required for maintenance of peripheral NKT cells in humans and mice. (A) The percentage of αGalCer-tetramer+ NKT in CD3+ PBMCs from healthy donors (closed circles) and DOCK8-deficient patients (open circles). Statistical significance was tested by the Mann-Whitney U test; *P < .05. (B) Representative plots of lymphocytes from the liver and spleen of WT, heterozygous (HET), and homozygous (CPM) DOCK8cpm/cpm mice, showing the gating strategy for NKT cells. (C) Absolute numbers of NKT cells in WT, DOCK8+/cpm, and DOCK8cpm/cpm mice (circles represent individual mice derived from 4 independent experiments) with statistical significance tested by 1-way ANOVA coupled with Dunn’s multiple comparison test; **P < .01; ***P < .001. (D) NK1.1, CD4, and CD44 expression on NKT cells from (left) WT (dark line) and DOCK8cpm/cpm mice (filled) and (right) absolute numbers of NK1.1+ and NK1.1− NKT subsets. Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by Mann-Whitney U test; *P < .05; **P < .01; ***P < .001. Flow cytometry plots are representative of 4 independent experiments.
DOCK8 is required for maintenance of peripheral NKT cells in humans and mice. (A) The percentage of αGalCer-tetramer+ NKT in CD3+ PBMCs from healthy donors (closed circles) and DOCK8-deficient patients (open circles). Statistical significance was tested by the Mann-Whitney U test; *P < .05. (B) Representative plots of lymphocytes from the liver and spleen of WT, heterozygous (HET), and homozygous (CPM) DOCK8cpm/cpm mice, showing the gating strategy for NKT cells. (C) Absolute numbers of NKT cells in WT, DOCK8+/cpm, and DOCK8cpm/cpm mice (circles represent individual mice derived from 4 independent experiments) with statistical significance tested by 1-way ANOVA coupled with Dunn’s multiple comparison test; **P < .01; ***P < .001. (D) NK1.1, CD4, and CD44 expression on NKT cells from (left) WT (dark line) and DOCK8cpm/cpm mice (filled) and (right) absolute numbers of NK1.1+ and NK1.1− NKT subsets. Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by Mann-Whitney U test; *P < .05; **P < .01; ***P < .001. Flow cytometry plots are representative of 4 independent experiments.
Comparison of peripheral NKT cell numbers in WT heterozygous DOCK8+/cpm and homozygous DOCK8cpm/cpm mice showed a three- to fourfold reduction of NKT cells in the peripheral tissues (liver, spleen, BM, and lymph nodes) of homozygous mutants (Figure 1B-C). Peripheral NKT cells from WT and DOCK8cpm/cpm mice expressed similar levels of the early developmental marker CD44 (Figure 1D), but disproportionately fewer DOCK8cpm/cpm NKT cells in the liver and spleen expressed NK1.1, a marker of peripheral differentiation, and CD4 (Figure 1D). Furthermore, the remaining NK1.1+ NKT cells expressed lower levels of this marker on the cell surface, particularly those in the liver (Figure 1D). These phenotypes were not different between WT and heterozygous DOCK8+/cpm mice, indicating these deficiencies were recessive (data not shown). Similar findings were observed in DOCK8pri/pri mice with equivalent losses of NKT cells and altered NK1.1 expression (supplemental Figure 2A). In conclusion, these findings show that loss of DOCK8 causes a deficiency in peripheral NKT cells, predominantly affecting differentiated NK1.1+ cells.
DOCK8 deficiency causes loss of the thymic resident CD44+NK1.1+ population
To investigate whether the loss of NKT cells could be explained by a block in their development, we compared WT and DOCK8-deficient cells in the thymus. Total numbers of thymic NKT cells were significantly reduced in DOCK8 mutant mice compared with WT (Figure 2A; supplemental Figure 2B). However, numbers of CD44−NK1.1− and semimature CD44+NK1.1− cells were comparable between the DOCK8 mutants and WT strains (Figure 2B; supplemental Figure 2C), implying that DOCK8 is not essential for early NKT cell development. These data also suggest that DOCK8 does not limit the production of migratory CD44+NK1.1− cells and that this is not the explanation for the loss of NKT cells in the periphery. In contrast, the larger resident CD44+NK1.1+ compartment of NKT cells was significantly reduced, and this accounted for the drop in total NKT cell numbers in the thymus (Figure 2B; supplemental Figure 2C).
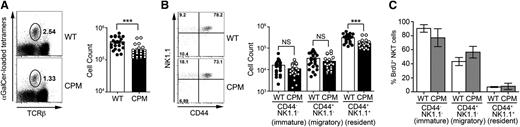
DOCK8 is required for maintenance of the thymic resident NK1.1+NKT cells. (A) Total TCRβ+αGalCer-tetramer+ thymic NKT cells in WT (closed circles) and DOCK8cpm/cpm (CPM, open circles) mice gated on CD8-negative live lymphocytes. (B) Total thymic TCRβ+αGalCer-tetramer+ NKT cells at different stages of development in WT (closed circles) and DOCK8cpm/cpm (CPM, open circles) mice. (C) BrdU labeling of thymic NKT cell subsets in WT (open columns) and DOCK8cpm/cpm (CPM, filled columns) mice (n = 5 for each) 9 days after its addition to drinking water. Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001. BrdU labeling data are representative of 3 independent experiments.
DOCK8 is required for maintenance of the thymic resident NK1.1+NKT cells. (A) Total TCRβ+αGalCer-tetramer+ thymic NKT cells in WT (closed circles) and DOCK8cpm/cpm (CPM, open circles) mice gated on CD8-negative live lymphocytes. (B) Total thymic TCRβ+αGalCer-tetramer+ NKT cells at different stages of development in WT (closed circles) and DOCK8cpm/cpm (CPM, open circles) mice. (C) BrdU labeling of thymic NKT cell subsets in WT (open columns) and DOCK8cpm/cpm (CPM, filled columns) mice (n = 5 for each) 9 days after its addition to drinking water. Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001. BrdU labeling data are representative of 3 independent experiments.
To address the possibility that a failure of homeostatic proliferation or increased turnover due to cell death could lead to NKT deficiency, we fed DOCK8cpm/cpm and WT mice bromodeoxyuridine (BrdU) for a period of 9 days. After this period, equivalent proportions of CD44−NK1.1− and CD44+NK1.1− NKT cells were labeled with BrdU, supporting the view that positive selection, the initial proliferation of NKT cell precursors, and generation of the migrating population were intact in DOCK8cpm/cpm mice (Figure 2C). In contrast, the equivalent proportion of labeled CD44+NK1.1+ cells in WT and DOCK8cpm/cpm, despite the difference in absolute numbers, implied that the differentiation of the resident cells or the survival of newly generated cells might be limited (Figure 2C). A significantly greater proportion of BrdU+ NKT cells in the liver of DOCK8cpm/cpm mice was consistent with higher turnover and decreased survival in the periphery (supplemental Figure 3A-B).
Cell intrinsic and extrinsic effects of DOCK8 deficiency on NKT cells
To distinguish between the direct and indirect effects of DOCK8 deficiency, we reconstituted irradiated WT recipient mice (CD45.1 allotype) with 80:20 mixtures of WT or DOCK8cpm/cpm (CD45.2 allotype) and WT cells (CD45.1 allotype). This ratio of DOCK8cpm/cpm to WT cells was chosen because past work has shown that DOCK8-deficient T cells fail to reconstitute the thymus to the same levels as WT cells.23,24 This phenotype was true of DOCK8cpm/cpm NKT cells with reconstitution levels of early NKT precursors (CD24hiαGalCer-tetramer+) at 66 ± 6.0% (mean ± standard error of the mean) compared with WT levels of 80 ± 2.3% (mean ± standard error of the mean; Figure 3A). In this more stringent test of the requirement of DOCK8 in NKT cell development, we found reduced levels of DOCK8cpm/cpm cells at the earliest stage of development after positive selection, when NKT cell precursors become CD24lo after engaging with antigen and SLAM receptors (Figure 3A). In addition, the loss of mature thymic NK1.1+ NKT cells persists in DOCK8cpm/cpm chimeric mice, demonstrating this is an intrinsic defect (Figure 3A).
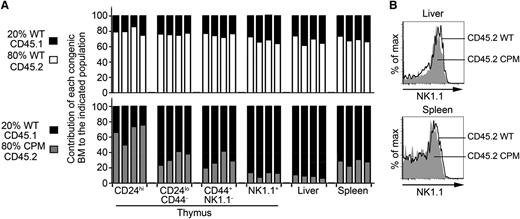
Cell intrinsic functions of DOCK8 regulate NKT cell selection and the number of NKT cells in the thymus and liver. (A) Mixed bone marrow chimeras generated by reconstituting irradiated CD45.1 allotype WT mice with BM derived from 80% WT CD45.2 or 80% DOCK8cpm/cpm (CPM) CD45.2 mice mixed with 20% CD45.1 WT BM. Bars show individual chimeric mice and reconstitution levels expressed as a percentage of allotype-specific αGalCer-tetramer+ NKT cells. Data are representative of 3 independent experiments using mixed chimeras. (B) Relative expression levels of the maturation marker NK1.1 on NKT cells in WT and DOCK8cpm/cpm (CPM) NKT cells from the liver and spleen representative of 4 biological replicates.
Cell intrinsic functions of DOCK8 regulate NKT cell selection and the number of NKT cells in the thymus and liver. (A) Mixed bone marrow chimeras generated by reconstituting irradiated CD45.1 allotype WT mice with BM derived from 80% WT CD45.2 or 80% DOCK8cpm/cpm (CPM) CD45.2 mice mixed with 20% CD45.1 WT BM. Bars show individual chimeric mice and reconstitution levels expressed as a percentage of allotype-specific αGalCer-tetramer+ NKT cells. Data are representative of 3 independent experiments using mixed chimeras. (B) Relative expression levels of the maturation marker NK1.1 on NKT cells in WT and DOCK8cpm/cpm (CPM) NKT cells from the liver and spleen representative of 4 biological replicates.
Because TCR signaling is required for the maturation of NKT cells,14 we examined the expression of NK1.1 on WT and DOCK8cpm/cpm cells. NK1.1 expression profiles were restored to normal levels on DOCK8-deficient NKT cells, suggesting that this defect in unmanipulated CPM mice is due to extrinsic factors (Figure 3B). Splenic DOCK8-deficient NKT cells were also restored to relatively normal levels, in comparison with their migratory, CD44+NK1.1−, precursor cells in the thymus (Figure 3A). In contrast to the spleen, the reduction in NKT cells in the liver persisted, confirming that this phenotype is principally cell intrinsic (Figure 3A). The exaggerated loss of DOCK8cpm/cpm liver NKT cells in mixed chimeras, despite relatively normal spleen NKT cells (compared with thymus) and normal NK1.1 expression, suggests that loss of the liver NKT cell population is due neither to a defect in thymic egress nor to a defect in differentiation to the CD44+NK1.1+ state.
NKT cell survival is diminished in the absence of DOCK8
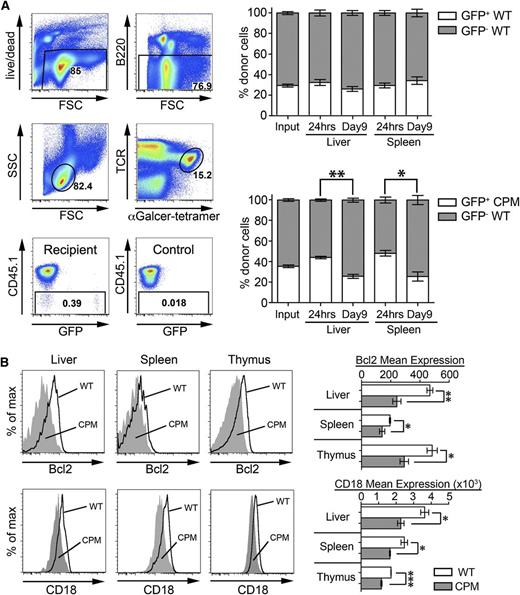
Current thinking suggests that the survival of mature NKT cells depends on growth factors including homeostatic cytokines, chemotactic factors, and environmental cues that they receive from cell-cell contact, notably via integrins.27-30 The survival response of WT and DOCK8cpm/cpm NKT cells from the thymus in culture with media supplemented with IL-2, IL-7, IL-15, or IL-21 was equivalent, suggesting that defective responses to soluble growth factors is not the cause of cell loss (supplemental Figure 4A). We therefore turned our attention to possible abnormalities in the migration, localization, and survival of NKT cells in vivo. We transferred mixtures of CD45.2 allotype and GFP-expressing WT and DOCK8cpm/cpm splenic NKT cells into WT (CD45.1 allotype) recipients for 24 hours and 9 days. After 24 hours, no deficiencies in the ability of DOCK8cpm/cpm NKT cells to migrate and localize to the liver and spleen were detectable (Figure 4A). However, when mice were analyzed 9 days after transfer, the proportion of DOCK8cpm/cpm NKT cells had fallen in both the liver and spleen (Figure 4A). DOCK8cpm/cpm NKT cells from the liver and thymus showed no increase in apoptosis by annexinV staining (supplemental Figure 4B). However, decreased expression of the prosurvival factor B-cell lymphoma 2 (Bcl-2) in NKT cells from unmanipulated DOCK8cpm/cpm animals supported the notion of an intrinsic survival defect (Figure 4B). The expression of the integrin lymphocyte function-associated antigen 1 (LFA-1) (CD11α/CD18), important for the survival and long-term retention of liver NKT cells,27,28 was also reduced in DOCK8cpm/cpm NKT cells in the liver and other organs (Figure 4B).
Decreased survival of NKT cells in the absence of DOCK8. (A) Adoptive transfer of mixed WT (CD45.2/GFP+) and WT or DOCK8cpm/cpm (CPM, CD45.2 GFP−) splenic NKT cells into WT (CD45.1) recipients. Gating strategy to define donor cells is illustrated showing recipient and control samples (left). Graphs show the proportion of CD45.2 GFP+ and GFP− NKT cells at input and after 24 hours and 9 days in the liver and spleen (right). Bars show arithmetic means based on 5 individual animals and error bars show standard error of the mean. Statistical significance was determined by the Mann-Whitney test with Kruskal-Wallis to correct for multiple comparisons; *P < .05; **P < .01. (B) Relative expression of Bcl-2 and CD18 on liver, spleen, and thymic NKT cells in WT (dark line) and DOCK8cpm/cpm (CPM, filled) mice, representative of 3 independent experiments including 5 animals. Data have been quantified as mean expression of Bcl-2 and CD18 (WT, white bars; CPM, gray bars). Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001.
Decreased survival of NKT cells in the absence of DOCK8. (A) Adoptive transfer of mixed WT (CD45.2/GFP+) and WT or DOCK8cpm/cpm (CPM, CD45.2 GFP−) splenic NKT cells into WT (CD45.1) recipients. Gating strategy to define donor cells is illustrated showing recipient and control samples (left). Graphs show the proportion of CD45.2 GFP+ and GFP− NKT cells at input and after 24 hours and 9 days in the liver and spleen (right). Bars show arithmetic means based on 5 individual animals and error bars show standard error of the mean. Statistical significance was determined by the Mann-Whitney test with Kruskal-Wallis to correct for multiple comparisons; *P < .05; **P < .01. (B) Relative expression of Bcl-2 and CD18 on liver, spleen, and thymic NKT cells in WT (dark line) and DOCK8cpm/cpm (CPM, filled) mice, representative of 3 independent experiments including 5 animals. Data have been quantified as mean expression of Bcl-2 and CD18 (WT, white bars; CPM, gray bars). Columns shows arithmetic mean, error bars show standard error of the mean, and statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001.
Loss of DOCK8 defines CD103+ NK1.1+ NKT cells in the thymus
We decided to take a molecular approach to elucidate the defect in survival by comparing gene expression in the NKT cell subsets using RNA microarrays. Analysis of the liver NKT cell phenotype was complicated by the difficulty of isolating cells and the greater difficulty in controlling for the different rate of generation of new residents. For these reasons, we examined the resident NK1.1+ population in the thymus.
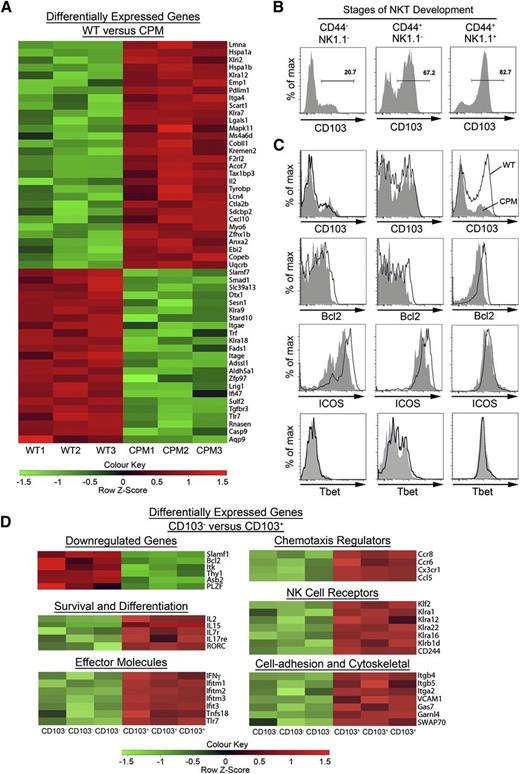
Comparison of TCRβ+αGalCer tetramer+CD8−CD44+NK1.1+ cells from the thymus of WT and DOCK8cpm/cpm mice showed differential expression of a relatively small subset of 52 genes (Figure 5A). Noticeable among these was the twofold lower overall expression of integrin αE (Itgae), also known as CD103, in DOCK8-deficient cells. CD103 binds integrin β7 to form the αEβ7 complex, whose main ligand is the epithelial cell adhesion molecule E-cadherin.31 CD103 has not previously been reported as a marker of NKT cells in the thymus but has been reported on NK1.1− NKT cells in the skin and peripheral lymph nodes.32 Flow cytometry of WT NKT cells in the thymus showed progressive up-regulation of CD103 expression during development (Figure 5B; supplemental Figure 5A), so that a majority of the most differentiated NK1.1+ cells expressed the integrin. Analysis of CD103 expression by NKT cell subsets in DOCK8-deficient mice revealed a selective loss of the CD103+ subset (Figure 5C). The prosurvival factor Bcl-2 was also reduced in the DOCK8cpm/cpm NK1.1+ NKT cells (Figure 5C; supplemental Figure 5B), whereas the expression of other molecules known to be associated with the development of this NK1.1+ NKT cell population, including inducible T-cell costimulator (ICOS) and T-box expressed in T cells (Tbet), were similar to WT counterparts in this subset (Figure 5C; supplemental Figure 5B).
DOCK8 maintains resident CD103+NK1.1+NKT T cells in the thymus. (A) Live TCRβ+αGalCer-tetramer+CD44+NK1.1+ NKT cells were flow-sorted to >99% purity from the thymuses of WT (n = 3) and DOCK8cpm/cpm (CPM, n = 3) mice, which had been depleted of CD8+ T cells by magnetic-activated cell sorting, and RNA from the cells was hybridized to the MouseWG-6-V2 microarray. The heat map shows 52 genes differentially expressed in thymic WT and DOCK8cpm/cpm (CPM) NK1.1+ T cells. (B) Expression of CD103 on αGalCer-tetramer+ NKT cells during development in the thymus in WT mice (representative of 3 independent experiments). (C) Expression of CD103, CD18, ICOS, and Tbet expression on WT (bold lines) and DOCK8cpm/cpm (CPM, filled) NKT cells during thymic development in mixed chimeras (reconstituted as before). (D) Live TCRβ+αGalCer-tetramer+CD44+NK1.1+ NKT cells were flow-sorted to >99% purity from WT mice on the basis of CD103 expression (as in panel B), with 3 biological replicates before RNA isolation and microarray analysis (as in panel A).
DOCK8 maintains resident CD103+NK1.1+NKT T cells in the thymus. (A) Live TCRβ+αGalCer-tetramer+CD44+NK1.1+ NKT cells were flow-sorted to >99% purity from the thymuses of WT (n = 3) and DOCK8cpm/cpm (CPM, n = 3) mice, which had been depleted of CD8+ T cells by magnetic-activated cell sorting, and RNA from the cells was hybridized to the MouseWG-6-V2 microarray. The heat map shows 52 genes differentially expressed in thymic WT and DOCK8cpm/cpm (CPM) NK1.1+ T cells. (B) Expression of CD103 on αGalCer-tetramer+ NKT cells during development in the thymus in WT mice (representative of 3 independent experiments). (C) Expression of CD103, CD18, ICOS, and Tbet expression on WT (bold lines) and DOCK8cpm/cpm (CPM, filled) NKT cells during thymic development in mixed chimeras (reconstituted as before). (D) Live TCRβ+αGalCer-tetramer+CD44+NK1.1+ NKT cells were flow-sorted to >99% purity from WT mice on the basis of CD103 expression (as in panel B), with 3 biological replicates before RNA isolation and microarray analysis (as in panel A).
To understand the factors affecting the development of CD103+ NKT cells, which might provide clues as to the role that DOCK8 plays in this process, we compared gene expression in CD103− vs CD103+ cells from WT mice (Figure 5D). The CD103+ subset was characterized by differential expression of 286 genes, with 220 genes expressed at higher levels by CD103+ NKT cells, whereas 66 genes were found at lower levels. CD103+ cells expressed high levels of genes involved in cell-cell contact and cytoskeletal regulation, including growth arrest specific gene 7 (Gas7), an adaptor protein involved the formation of neurites through direct interactions with actin and an association with N–Wiskott-Aldrich syndrome protein (WASP).33 The mRNA of the actin binding protein, SWAP70, was also increased threefold in CD103+ cells. SWAP70 deficiency results in the loss of germinal center and MZ B cells34 and impaired activation of LFA-1 in B- and T-cell synapse formation similar to that already reported for DOCK8cpm/cpm and DOCK8pri/pri strains. Thus, proteins such as Gas7 and SWAP70 may be part of signaling pathways downstream of DOCK8 activation. On the other hand, CD103 itself is not essential for the survival of the resident NKT cells in the thymus, because normal numbers of NK1.1+ NKT cells are present in CD103-deficient mice (supplemental Figure 6).
Activation with αGalCer demonstrates intact TCR signaling but defective proliferative responses
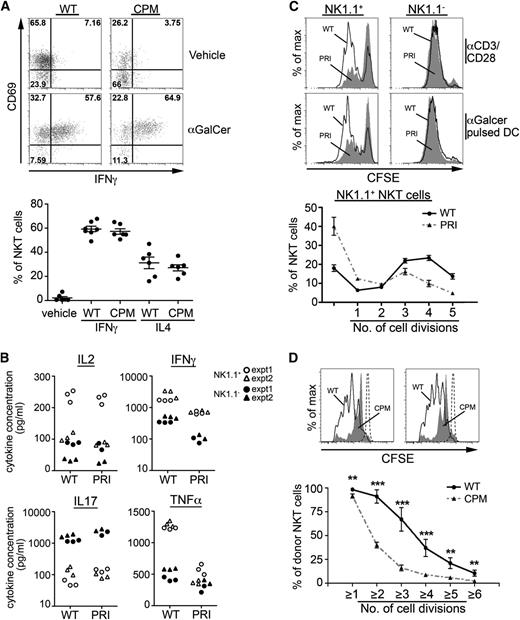
Because previous studies in DOCK8-deficient mice have shown defects in formation of the B- and T-cell immune synapses,22,24 we asked if abnormalities in antigen presentation or response could account for decreased survival of the peripheral DOCK8-deficient NKT cells. Accordingly, we injected DOCK8cpm/cpm mice with αGalCer and looked at NKT activation by intracellular cytokine staining. Despite the reduction in liver NKT cells, the responses were equivalent in DOCK8cpm/cpm mice compared with WT, suggesting TCR signaling is unaffected by the loss of DOCK8 function and that processes such as antigen presentation were not limiting (Figure 6A). However, responses in the spleen were significantly weaker (supplemental Figure 7A). We speculated that this defect might be due to the absence of CD1dhi MZ B cells,22 which have previously been shown to play a role in NKT cell activation in the spleen.35 In agreement with this hypothesis, cytokine responses were restored in splenic DOCK8cpm/cpm NKT cells in mixed chimeras (supplemental Figure 7B).
DOCK8 is necessary for normal NKT effector responses: intact TCR signaling but defective antigen-induced proliferation and cytokine production. (A) WT and DOCK8cpm/cpm (CPM) mice were examined for cytokine responses 1 hour after injection with αGalCer by staining for CD69 and IFNγ and IL-4 staining by intracellular cytokine staining (upper). Graph (lower) showing the percentage of IFNγ or IL-4 secreting NKT cells in individual mice (circles) with arithmetic means and standard error of the mean. (B) Levels of secreted cytokines from 24-hour cultures of sorted thymic NK1.1+ and NK1.1− NKT cells from WT and DOCK8pri/pri (PRI) mice activated in vitro with plate bound anti-CD3/CD28. Symbols represent replicate measurements with data from 2 independent experiments. (C) Proliferation of CFSE-labeled thymic NK1.1+ and NK1.1− NKT cells sorted from DOCK8pri/pri (PRI, filled) and WT mice (WT, black line) and cultured for 3 days with plate bound anti-CD3/CD28 and αGalCer pulsed dendritic cells. Cell divisions from replicate cultures from 1 experiment with dendritic cells (lower) show arithmetic means and standard deviation. (D) Proliferation of CFSE-labeled thymic NKT cells from WT and DOCK8cpm/cpm (CPM) mice adoptively transferred into WT mice and immunized with 1 μg αGalCer per mouse. Histograms (top) show NKT cells from WT (black line), DOCK8cpm/cpm (CPM, filled), and untreated (dotted line) mice after 4 days. Cell divisions (bottom) show arithmetic means and standard deviation. Statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001.
DOCK8 is necessary for normal NKT effector responses: intact TCR signaling but defective antigen-induced proliferation and cytokine production. (A) WT and DOCK8cpm/cpm (CPM) mice were examined for cytokine responses 1 hour after injection with αGalCer by staining for CD69 and IFNγ and IL-4 staining by intracellular cytokine staining (upper). Graph (lower) showing the percentage of IFNγ or IL-4 secreting NKT cells in individual mice (circles) with arithmetic means and standard error of the mean. (B) Levels of secreted cytokines from 24-hour cultures of sorted thymic NK1.1+ and NK1.1− NKT cells from WT and DOCK8pri/pri (PRI) mice activated in vitro with plate bound anti-CD3/CD28. Symbols represent replicate measurements with data from 2 independent experiments. (C) Proliferation of CFSE-labeled thymic NK1.1+ and NK1.1− NKT cells sorted from DOCK8pri/pri (PRI, filled) and WT mice (WT, black line) and cultured for 3 days with plate bound anti-CD3/CD28 and αGalCer pulsed dendritic cells. Cell divisions from replicate cultures from 1 experiment with dendritic cells (lower) show arithmetic means and standard deviation. (D) Proliferation of CFSE-labeled thymic NKT cells from WT and DOCK8cpm/cpm (CPM) mice adoptively transferred into WT mice and immunized with 1 μg αGalCer per mouse. Histograms (top) show NKT cells from WT (black line), DOCK8cpm/cpm (CPM, filled), and untreated (dotted line) mice after 4 days. Cell divisions (bottom) show arithmetic means and standard deviation. Statistical significance was tested by unpaired t test; *P < .05; **P < .01; ***P < .001.
To investigate NKT cell responses to antigen in more detail and to compare immature and mature NKT cell populations, we sorted NK1.1− and NK1.1+ NKT cells from the thymus of WT and DOCK8pri/pri mice and stimulated them with plate-bound anti-CD3/CD28 for 24 hours before collection of supernatant. Although both NK1.1+ and NK1.1− subsets of NKT cells secreted IFNγ and tumor necrosis factor α, the NK1.1+ subset produced greater quantities than the NK1.1− subset. In both cases, this was reduced in the absence of DOCK8 (Figure 6B). In contrast, IL-2 production by both NK1.1+ and NK1.1− cells—more in the former—showed no dependence on DOCK8, and IL-17 was produced exclusively by NK1.1− cells in a DOCK8-independent manner. The proliferation of mature DOCK8pri/pri NK1.1+ NKT cells, but not NK1.1− cells, as indicated by dilution of CFSE labeling, was significantly reduced in response to anti-CD3/CD28 stimulation and in response to αGalCer (Figure 6C). To assess proliferation in vivo, CFSE-labeled WT and DOCK8cpm/cpm NKT cells were transferred into WT recipients and stimulated with αGalCer. Tissues were harvested after 4 days, and NKT cells were examined for evidence of proliferation. Here, defective activation of DOCK8-deficient NKT cells was also illustrated by reduced proliferation in vivo (Figure 6D).
Together, these results show that the NKT cell deficiency in the absence of DOCK8 appears to be due to an intrinsic defect in the rate of formation and survival of the most differentiated mature cells and a failure of these cells to maintain proliferation and cytokine production on activation.
Discussion
In this study, we described how deficiency in the GEF DOCK8 leads to reduced numbers and activation of mature CD44+NK1.1+ NKT cells. Although DOCK8 is not absolutely required for NKT cell activation, it does appear to be necessary for normal differentiation of the CD44+NK1.1+ subset and maintenance of proliferative responses. The deficiency in proliferative responses appears to be specific to mature NKT cells, as previous work in our laboratories has demonstrated intact proliferative responses in other cell types including naïve T cells23,24 and follicular B cells.22 Therefore, the reduced survival of the mature NKT cells in the resting and proliferating state in the absence of DOCK8 is expected to have significant effects on innate and adaptive immune responses.
Reduced numbers of effector NKT cells may contribute to the susceptibility to infectious disease and cancer seen in the human DOCK8 immunodeficiency. Several immunodeficiency syndromes have been associated with NKT cell deficiency such as Wiskott-Aldrich syndrome,36,37 Omenn’s syndrome,38 and X-linked lymphoproliferative (XLP) disease.39 NKT cells play an important role in the immediate response to pathogens through mechanisms including the release of inflammatory cytokines and activation of innate immune cells such as NK cells.40 Therefore, the absence and impaired function of NKT cells in DOCK8 deficiency is likely to affect the quality and amplitude of immunity to the recurrent infections that beset these patients. There is also compelling evidence that NKT cells play a role in cancer surveillance.5 Interestingly, low levels of circulating NKT cells are associated with poor tumor suppression and disease-specific survival rates in patients with head and neck squamous cell carcinoma41 ; in mice, NKT cells from the liver provide the best protection against cancer compared with NKT cells from the spleen or thymus.42
The earliest NKT cell progenitors arise from CD4+CD8+ DP thymocytes carrying the invariant NKT cell receptor. These cells undergo positive selection after interaction with an unidentified CD1d/lipid self-antigen in conjunction with costimulation via SLAM receptors.43 The SLAM receptors signal via the adaptor protein signaling lymphocyte activation molecule-associated protein and the Src family kinase Fyn.44 Deficiency in signaling lymphocyte activation molecule-associated protein causes XLP disease, which is characterized by a block in NKT cell development at positive selection, an inability to control Epstein-Barr virus infection, and a failure to generate humoral immunity.45,46 There are parallels between XLP and DOCK8 deficiency, with defects in both B- and NKT-cell immunity, as well as viral susceptibility and an increased incidence of B lymphoma.20 As previously indicated, a related DOCK protein DOCK2 is required for NKT cell development. DOCK2-deficient mice show a selective loss of NKT cells during positive selection.21 However, although DOCK8 deficiency has a marked effect on the positive selection of NKT cells in the presence of competing WT cells, it does not appear to be limiting in the absence of competition or in vitro in response to TCR stimuli.
In unmanipulated DOCK8-deficient mice, the principal effect of loss of DOCK8 is the reduction in the resident CD44+ NK1.1+ population in the thymus. The selection and coincident proliferation of the CD44+NK1.1− precursors leading to the formation of this population relies on a number of factors including antigen presentation by CD1d, engagement of SLAM family receptors, interactions of costimulatory molecules such as B7-CD28 and ICOS–ICOS-ligand, and expression of transcription factors including promyelocytic leukaemia zinc finger and Tbet, normally leading to up-regulation of CD122, which has an essential role in cell survival.11,13,47-49 Expression of these factors was similar in WT and DOCK8-deficient CD44+NK1.1+ NKT cells, based on gene array analysis, implying they are not differentially regulated at the level of transcription.
Survival and homeostatic proliferation of the CD44+NK1.1+ NKT cells is independent of CD1d signaling but does require IL-15.14,29 Although we demonstrate that DOCK8 deficiency interferes with the proliferative capacity of NK1.1+ NKT cells in response to TCR stimuli, normal incorporation of BrdU into the CD44+NK1.1+ compartment in vivo suggests proliferation during the late stages of development is normal. Consistent with this, DOCK8-deficient cells respond normally to IL-15 stimulation in vitro, suggesting that DOCK8 operates in a separate survival pathway. Because DOCK family members are recruited to the plasma membrane by binding to phosphatidylinositol phosphate (PIP)2/PIP3, such a prosurvival pathway could relate to the canonical PIP3-dependent AKT- and mitogen-activated protein kinase–dependent cell survival pathways.
The patterns of NKT cell development and survival seen in the thymus are repeated in the periphery where maturation and survival are dependent on antigen presentation and homeostatic cytokines.13 Although differentiation of the NK1.1+ subset remains CD1d dependent, NKT cell survival does not appear to require TCR/CD1d interactions. Our findings have shown that the initial activation of NKT cells is normal in the liver and in the spleen in the presence of WT hematopoietic cells including CD1d-positive MZ B cells. This suggests that TCR/CD1d interactions and signaling are broadly intact and unlikely to explain the deficiency of NKT cells in the periphery. Because the numbers of splenic NKT cells are not reduced in mixed BM chimeras, the cell intrinsic defect in NKT cell survival in the liver appears to be more specific.
Like MZ B cells, which are absent in DOCK8 deficiency, NKT cells depend on signals from integrins including LFA-1 for tissue retention and survival.28 It is therefore possible that survival signals downstream of integrin receptors play a key role in NKT cell survival in the thymus and periphery.27,50 In this study, we identified the integrin CD103 as a novel marker of NKT cell development in the thymus. Although expression of CD103 is reduced in the absence of DOCK8, it is not absolutely required for maintenance of thymic NKT cells. Loss of the integrin LFA-1 using knockout mice has demonstrated that the dependence of NKT cells on LFA-1 is predominantly in the liver, with no differences in splenic NKT cells.27,50 WASP-deficient NKT cells are reduced in the liver, where their deficiency is associated with decreased expression of LFA-1.37 In relation to this, the importance of LFA-1 in NKT cell homeostasis has been demonstrated in integrin blocking studies using parabiotic mice.28 Similar to WASP-deficient NKT cells, alterations in LFA-1 expression and cytoskeletal regulators results in impaired proliferative responses in response to αGalCer.36,37 Further analysis of the functionality of integrins in DOCK8-deficient cells will be required to confirm such hypotheses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participating in this study, members of our laboratories for review of the manuscript, and the staff of the Australian Phenomics Facility and Oxford Biomedical Services Unit for excellent animal husbandry.
This work was supported by the Medical Research Council, National Institute for Health Research Biomedical Research Centre Program, National Institutes of Medical Research, Australian National Health and Medical Research Council (NHMRC), and the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. The authors acknowledge the following personal awards: Medical Research Council Centenary Early Career Award (to G.C.), NHMRC Career Development Fellowship (to A.E.), NHMRC Senior Principal Research Fellowship (to D.I.G.), NHMRC Career Development Fellowship (to C.S.M.), NHMRC grant GNT1022922 (to K.L.R.), and NHMRC Principal Research Fellowship (to S.G.T.).
Authorship
Contribution: G.C., A.E., U.G., S.S., Q.Z., T.L., T.L.C., H.E.L., and C.S.M. designed and performed experiments and analyzed and interpreted data; A.F., J.M.S., and P.D.A. provided patient samples; S.G.T., C.C.G., V.C., D.I.G., H.C.S., K.L.R., and R.J.C. designed experiments, analyzed and interpreted data, and performed statistical analysis; and G.C., K.L.R., and R.J.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Cornall, MRC Human Immunology Unit, Weatherall Institute for Molecular Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom; e-mail: richard.cornall@ndm.ox.ac.uk.
References
Author notes
G.C., A.E., and U.G. contributed equally to this study.
K.L.R. and R.J.C. contributed equally to this study.