Key Points
Panobinostat + bortezomib + dexamethasone recaptures responses in heavily pretreated, bortezomib-refractory multiple myeloma patients.
Abstract
Panobinostat is an oral pan-deacetylase inhibitor that synergizes with bortezomib to inhibit both the aggresome and proteasome pathways in preclinical studies. PANORAMA 2 is a phase 2 trial of panobinostat in combination with bortezomib and dexamethasone to treat patients with relapsed and bortezomib-refractory multiple myeloma (with ≥2 prior lines of therapy, including an immunomodulatory drug, and patients who had progressed on or within 60 days of the last bortezomib-based therapy). Fifty-five heavily pretreated patients were enrolled (median, 4 prior regimens, including a median of 2 prior bortezomib-containing regimens). The overall response rate was 34.5% (1 near-complete response and 18 partial responses). An additional 10 patients achieved minimal response, for a clinical benefit rate of 52.7%. Median exposure and progression-free survival were 4.6 and 5.4 months, respectively. In patients who achieved a response, median time to response was 1.4 months, and median duration of response was 6.0 months. Common grade 3/4 adverse events, regardless of study drug relationship, included thrombocytopenia (63.6%), fatigue (20.0%), and diarrhea (20.0%). Only 1 patient had grade 3 peripheral neuropathy. Panobinostat, when combined with bortezomib and dexamethasone, can recapture responses in heavily pretreated, bortezomib-refractory multiple myeloma patients. This trial was registered at www.clinicaltrials.gov as #NCT01083602.
Introduction
Although frontline treatment of multiple myeloma (MM) is often associated with high response rates,1-4 the disease remains largely incurable, and nearly all patients eventually relapse. As patients relapse and become refractory to commonly used agents and regimens—including the proteasome inhibitor bortezomib and the immunomodulatory drugs (IMiDs) lenalidomide and thalidomide—their prognosis worsens.1,2,5-8 This is particularly true for patients with MM that is refractory to bortezomib and relapsed, and/or are refractory, intolerant, or ineligible to receive an IMiD. These patients have a median overall survival (OS) and event-free survival (EFS) of 9 and 5 months, respectively, and only 20% achieve responses on subsequent bortezomib-containing regimens.9 Thus, novel agents that can recapture responses in bortezomib-refractory MM patients are needed.
Panobinostat is a pan-deacetylase inhibitor with low nanomolar activity against all class I, II, and IV histone deacetylase enzymes, including those implicated as potential targets in MM.10 Panobinostat increases acetylation of proteins involved in multiple oncogenic pathways, including the aggresome protein degradation pathway.10 In preclinical studies, inhibition of the aggresome and proteasome pathways by panobinostat and bortezomib, respectively, led to synergistic cytotoxicity against MM cells.11
Panobinostat was previously investigated in a phase 1b dose escalation/expansion study in patients with relapsed or relapsed/refractory MM.12 In combination with bortezomib and dexamethasone (at the investigator’s discretion beginning at cycle 2), the maximum tolerated dose of panobinostat was 20 mg 3 times per week every week in the dose escalation phase. In the expansion phase of that study, dexamethasone was required beginning at cycle 2, and the schedule was amended to introduce a rest week for panobinostat (ie, 2 weeks on, 1 week off). This schedule achieved similar efficacy and suggested better tolerability compared with the weekly dosing schedule. Specifically, lower rates of hematologic toxicities and a longer duration of therapy were observed. The combination was also efficacious, achieving an overall response rate (ORR) of 55% in all patients and a 37% ORR among bortezomib-refractory patients.
On the basis of these intriguing preclinical mechanistic data and promising early-phase clinical trial results, PANORAMA 2 sought to evaluate the combination of panobinostat with bortezomib and dexamethasone in patients with bortezomib-refractory MM.
Patients and methods
Study design and objectives
PANORAMA 2 is a phase 2, two-stage, single-arm, open-label multicenter study of oral panobinostat in combination with bortezomib and dexamethasone. The primary objective was to evaluate ORR (as defined by the modified European Group for Blood and Marrow Transplantation [EBMT] criteria13 ), and secondary objectives included evaluation of minimal response (MR), time to response, duration of response (DoR), progression-free survival (PFS), OS, and safety and tolerability of the combination. Very good partial response (VGPR) was assessed based on International Myeloma Working Group (IMWG) criteria.14,15
This trial was registered at www.clinicaltrials.gov as #NCT01083602. The study was conducted according to the ethical principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice. The protocol and amendments were approved by the institutional review board or independent ethics committee for each center. All patients provided written informed consent before study entry.
Patients
Adult patients ≥18 years of age with relapsed and bortezomib-refractory MM (progressed on or within 60 days of the last bortezomib-containing regimen) who had received at least 2 prior lines of therapy and had been exposed to an IMiD were eligible for the trial. Patients were required to have measurable disease, defined as M protein ≥10 g/L or urine M protein ≥200 mg per 24 hours, based on IMWG 2003 definitions.16 Eligible patients were also required to have an Eastern Cooperative Oncology Group performance status ≤2, absolute neutrophil count ≥1.0 × 109/L, and platelet count ≥70 × 109/L. In addition, electrolyte levels were required to be within normal limits and transaminase levels had to be ≤2.5 × the upper limit of normal.
Patients with primary refractory disease, prior MM therapy with a DACi, history of allogeneic stem cell transplant with active graft-versus-host disease requiring immunosuppressive therapy, and/or peripheral grade ≥2 were excluded from the trial.
Treatment schedule
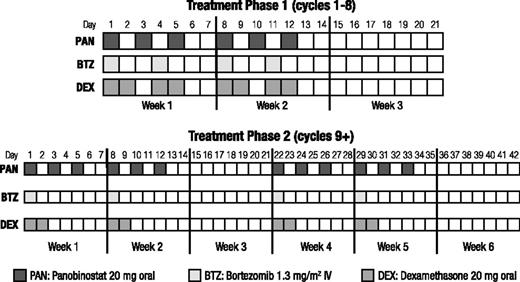
Patients were treated in 2 phases (Figure 1), both with a 2-week-on/1-week-off schedule. Phase 1 treatment consisted of 8 three-week cycles of oral panobinostat (20 mg) 3 times per week on weeks 1 and 2, bortezomib (1.3 mg/m2 intravenously) 2 times per week on weeks 1 and 2, and oral dexamethasone (20 mg) 4 times per week on weeks 1 and 2 on days of and after bortezomib use. Patients who showed evidence of clinical benefit in phase 1 treatment continued study therapy in phase 2 treatment, which consisted of 6-week cycles of panobinostat 3 times per week on weeks 1, 2, 4, and 5; bortezomib once per week on weeks 1, 2, 4, and 5; and dexamethasone on the days of and after bortezomib until disease progression, death, toxicity, or withdrawal of consent.
Dosing schedule. During treatment phase 1 (cycles 1-8), panobinostat (3×/week), bortezomib (2×/week), and dexamethasone (the day of and after bortezomib) were administered on a 2-weeks-on/1-week-off schedule. Treatment phase 2 (cycles 9+) consisted of 6-week cycles (two 2-weeks-on/1-week-off repeating cycles), with bortezomib and the corresponding dexamethasone reduced to once per week during their scheduled weeks. IV, intravenous.
Dosing schedule. During treatment phase 1 (cycles 1-8), panobinostat (3×/week), bortezomib (2×/week), and dexamethasone (the day of and after bortezomib) were administered on a 2-weeks-on/1-week-off schedule. Treatment phase 2 (cycles 9+) consisted of 6-week cycles (two 2-weeks-on/1-week-off repeating cycles), with bortezomib and the corresponding dexamethasone reduced to once per week during their scheduled weeks. IV, intravenous.
Assessments
Data are presented as of February 20, 2012. Disease assessments were based on modified EBMT 1998 criteria,13 with the exploratory objective of VGPR determined by IMWG 2008 uniform criteria.14,15 Disease assessments—performed every 3 weeks, with responses confirmed after 6 weeks—entailed measurements of serum M protein (serum protein electrophoresis), urine M protein (urine protein electrophoresis), serum-free light chains, immunoglobulins (serum immunofixation), urine proteins (urine immunofixation), and evaluation of soft tissue plasmacytomas. In addition, bone marrow aspirate for plasma cell count was assayed at screening and for complete response (CR) confirmation, and skeletal surveys were performed at screening and during the study if they were clinically indicated. Patients who discontinued study treatment for reasons other than documented disease progression continued to have disease assessments performed every 6 weeks until documented disease progression or death. After disease progression, patients were followed every 3 months for survival for up to 2 years.
Safety was monitored throughout the trial and up to 28 days after the last dose of study treatment. Adverse events (AEs) were assessed according to Common Terminology Criteria for Adverse Events version 4.0. Electrocardiogram monitoring was performed throughout the first 8 cycles. The Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity subscale version 4.0 was used to determine the presence and intensity of neuropathic pain and/or peripheral neuropathy at baseline, day 1 of cycle 1, and every 6 weeks thereafter.
Statistical analyses
The primary efficacy variable was overall response rate, defined as the proportion of patients with a best overall disease response of better than or equal to partial response (PR; CR or near-CR or PR). Sample size was estimated based on a 2-stage design to test the null hypothesis of a response rate of P ≤ 10% vs an alternative hypothesis of a response rate of P > 10%. Using a 1-sided type I error rate of 0.05 and 80% power, 47 evaluable patients were required for the study. At least 4 responses were required in stage 1 (n = 24) to enroll an additional 23 patients in stage 2, and at least 9 of 47 patients at the end of stage 2 were needed to reject the null hypothesis. Additional statistical tests were performed at a 2-sided significance level of .05. Point estimates and exact 95% confidence intervals were calculated for response rates, and Kaplan-Meier estimates were used to summarize PFS and OS. All authors had access to the primary trial data.
Results
Patient characteristics and disposition
Fifty-five patients were enrolled in the trial from June 2010 to July 2011 at 12 sites. Baseline demographics and other characteristics are presented in Table 1. Patients were heavily pretreated and received multiple prior regimens (median, 4 [range, 2-11]), including multiple prior bortezomib-based therapies (median, 2 [range, 1-6]). Forty patients (72.7%) had progressed while on their last bortezomib regimen, and 15 patients (27.3%) had progressed within 60 days after their last bortezomib regimen. Median cumulative duration of prior bortezomib treatment was 8.7 months (range, 1.6-42.6) in the 54 patients with available data. The starting dose of bortezomib administered in their most recent bortezomib-containing regimen was 1.0 mg/m2 in 9 patients and 1.3 mg/m2 in 22 patients; 24 patients had received other doses ranging from 0.7 to 3.5 mg/m2 with once- or twice-weekly schedules, sometimes with rest weeks. Twenty-seven patients (49.1%) received bortezomib and 37 patients (67.3%) received dexamethasone in their most recent prior line of therapy. Forty-five patients (81.8%) received dexamethasone in a prior bortezomib-containing regimen, with a dexamethasone dose of <20 mg in 9 patients, ≥20 and <40 mg in 14 patients, and ≥40 mg in 11 patients (9 patients received doses that were not recorded). All except 1 patient had received prior lenalidomide (n = 54; 98.2%), 38 patients (69.1%) had received prior thalidomide, and 37 patients (67.3%) had received both lenalidomide and thalidomide. With the most recent prior regimen, 21.8% of all patients achieved a response (1 CR [1.8%] and 11 PR [20.0%]). Most patients (n = 37; 67.3%) reported neuropathy with a prior line of therapy. The highest grade of neuropathy in any prior bortezomib-containing regimen was primarily grade 1 (n = 16), with grades 2, 3, and 4 events reported in 3, 1, and 1 patients, respectively. The grades of the remaining 16 patients’ events were unknown.
Patient demographics, baseline characteristics, and disease history
| . | N = 55 . |
|---|---|
| Female/male, n (%) | 26/29 (47.3/52.7) |
| Median age, y (range) | 61 (41-88) |
| Age ≥65 y, n (%) | 21 (38.2) |
| ECOG performance status, n (%) | |
| 0 | 26 (47.3) |
| 1 | 25 (45.5) |
| 2 | 4 (7.3) |
| Baseline serum albumin (g/L), median (range) | 36.9 (30.6-48.9) |
| Baseline serum M protein (g/L), median (range) | 26.0 (0-66.0) |
| Baseline urine M protein (mg/24 h), median (range) | 283 (0-9628) |
| ISS staging, n (%) | |
| Stage 1 | 18 (32.7) |
| Stage 2 | 23 (41.8) |
| Stage 3 | 13 (23.6) |
| Missing* | 1 (1.8) |
| Immunoglobulin subtype, n (%) | |
| IgG | 35 (63.6) |
| IgA | 12 (21.8) |
| IgM | 1 (1.8) |
| Indeterminate | 7 (12.7) |
| Light-chain subtype, n (%) | |
| κ | 37 (67.3) |
| Λ | 16 (29.1) |
| Indeterminate | 2 (3.6) |
| FISH, n (%) | |
| Normal | 2 (3.6) |
| Any abnormality† | 35 (63.6) |
| del(17p), t(4;14), or t(14;16) | 14 (25.5) |
| del(13q) | 5 (9.1) |
| t(11;14) | 14 (25.5) |
| 3+ | 1 (1.8) |
| Median time since diagnosis, months (range) | 54.8 (7.5-263.6) |
| Prior regimens, median (range) | 4 (2-11) |
| Prior therapy, n (%) | |
| BTZ | 55 (100.0) |
| DEX | 55 (100.0) |
| Lenalidomide | 54 (98.2) |
| Thalidomide | 38 (69.1) |
| Prior autologous stem cell transplant, n (%) | 31 (56.4) |
| Median cumulative duration of prior BTZ‡, months (range) | 8.7 (1.6-42.6) |
| Prior BTZ regimens, median (range) | 2 (1-6) |
| Progressed while on last BTZ regimen, n (%) | 40 (72.7) |
| Progressed ≤60 d after last BTZ regimen, n (%) | 15 (27.3) |
| BTZ in most recent prior regimen, n (%) | 27 (49.1) |
| DEX in most recent prior regimen, n (%) | 37 (67.3) |
| DEX in last BTZ-containing regimen, n (%) | 45 (81.8) |
| Best response at last treatment, n (%) | |
| Complete response | 1 (1.8) |
| Partial response | 11 (20.0) |
| Minimal response | 10 (18.2) |
| Stable disease | 8 (14.5) |
| Progressive disease | 17 (30.9) |
| None | 5 (9.1) |
| Unknown | 3 (5.5) |
| . | N = 55 . |
|---|---|
| Female/male, n (%) | 26/29 (47.3/52.7) |
| Median age, y (range) | 61 (41-88) |
| Age ≥65 y, n (%) | 21 (38.2) |
| ECOG performance status, n (%) | |
| 0 | 26 (47.3) |
| 1 | 25 (45.5) |
| 2 | 4 (7.3) |
| Baseline serum albumin (g/L), median (range) | 36.9 (30.6-48.9) |
| Baseline serum M protein (g/L), median (range) | 26.0 (0-66.0) |
| Baseline urine M protein (mg/24 h), median (range) | 283 (0-9628) |
| ISS staging, n (%) | |
| Stage 1 | 18 (32.7) |
| Stage 2 | 23 (41.8) |
| Stage 3 | 13 (23.6) |
| Missing* | 1 (1.8) |
| Immunoglobulin subtype, n (%) | |
| IgG | 35 (63.6) |
| IgA | 12 (21.8) |
| IgM | 1 (1.8) |
| Indeterminate | 7 (12.7) |
| Light-chain subtype, n (%) | |
| κ | 37 (67.3) |
| Λ | 16 (29.1) |
| Indeterminate | 2 (3.6) |
| FISH, n (%) | |
| Normal | 2 (3.6) |
| Any abnormality† | 35 (63.6) |
| del(17p), t(4;14), or t(14;16) | 14 (25.5) |
| del(13q) | 5 (9.1) |
| t(11;14) | 14 (25.5) |
| 3+ | 1 (1.8) |
| Median time since diagnosis, months (range) | 54.8 (7.5-263.6) |
| Prior regimens, median (range) | 4 (2-11) |
| Prior therapy, n (%) | |
| BTZ | 55 (100.0) |
| DEX | 55 (100.0) |
| Lenalidomide | 54 (98.2) |
| Thalidomide | 38 (69.1) |
| Prior autologous stem cell transplant, n (%) | 31 (56.4) |
| Median cumulative duration of prior BTZ‡, months (range) | 8.7 (1.6-42.6) |
| Prior BTZ regimens, median (range) | 2 (1-6) |
| Progressed while on last BTZ regimen, n (%) | 40 (72.7) |
| Progressed ≤60 d after last BTZ regimen, n (%) | 15 (27.3) |
| BTZ in most recent prior regimen, n (%) | 27 (49.1) |
| DEX in most recent prior regimen, n (%) | 37 (67.3) |
| DEX in last BTZ-containing regimen, n (%) | 45 (81.8) |
| Best response at last treatment, n (%) | |
| Complete response | 1 (1.8) |
| Partial response | 11 (20.0) |
| Minimal response | 10 (18.2) |
| Stable disease | 8 (14.5) |
| Progressive disease | 17 (30.9) |
| None | 5 (9.1) |
| Unknown | 3 (5.5) |
BTZ, bortezomib; del, deletion; DEX, dexamethasone; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; Ig, immunoglobulin; ISS, International Staging System; t, translocation.
One patient did not have baseline β2 microglobulin measurement.
Not all subcategories of abnormalities are presented, and patients could present with more than 1 abnormality.
One patient had missing data.
Seventeen of the 55 patients completed treatment phase 1 and entered treatment phase 2. At the time of data cutoff, 7 of these 17 patients remained on treatment. The primary reasons for discontinuing treatment (n = 48) were disease progression (n = 31; 56.4%), AE (n = 10; 18.2%), withdrawal of consent (n = 5; 9.1%), death (n = 1; 1.8%), and start of a new cancer therapy (n = 1; 1.8%). The most common AEs leading to study treatment discontinuation were fatigue (n = 4), diarrhea (n = 2), asthenia (n = 2), and pneumonia (n = 2). One patient died during study treatment and 3 others died within 28 days of study treatment (3 deaths from disease progression/MM and 1 from influenza). None of the deaths were assessed as being study treatment related.
Median exposure was 4.6 months (range, 0.1-14.8). Two patients completed ≥12 cycles (48 weeks) of treatment. Dose reductions of panobinostat, bortezomib, and dexamethasone occurred in 35 (63.6%), 36 (65.5%), and 15 (27.3%) patients, respectively. Dose interruptions of panobinostat, bortezomib, and dexamethasone occurred in 32 (58.2%), 27 (49.1%), and 40 (72.7%) patients, respectively. Median relative dose intensity was 72.9% for panobinostat. The median relative dose intensities for bortezomib and dexamethasone were 79.8% and 87.5%, respectively. The most common AEs requiring dose adjustment or study treatment interruption, regardless of study drug relationship, were thrombocytopenia, fatigue, diarrhea, and vomiting.
Efficacy
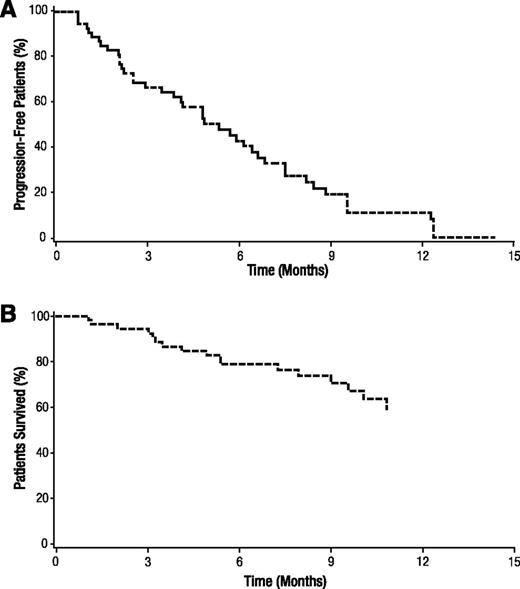
More than 4 responses were observed in stage 1, and the study continued to stage 2. In this population of bortezomib-refractory patients, 1 patient (1.8%) achieved a near-complete response, and 18 patients (32.7%) achieved a PR, for an ORR of 34.5% (≥PR; Table 2). An additional 10 patients (18.2%) achieved an MR, bringing the clinical benefit rate (CBR; ≥MR) to 52.7%. In an exploratory analysis, 3 patients (5.5%) achieved a VGPR. Responses in the remainder of patients consisted of stable disease in 20 patients (36.4%) and progressive disease in 3 patients (5.5%). Response could not be assessed in the remaining 3 patients (5.5%) because of insufficient or missing assessments. In the 19 patients who achieved a response (≥PR), median time to response was 1.4 months and median DoR was 6.0 months. For all patients, median PFS was 5.4 months (Figure 2A). With a median follow-up of 8.3 months, median OS has not yet been reached (Figure 2B).
Best response (confirmed at 6 weeks) at the end of 8 cycles
| . | N = 55 . |
|---|---|
| n (%) . | |
| Overall response (at least partial response) | 19 (34.5) |
| Complete response | 0 |
| Near-complete response | 1 (1.8) |
| Partial response | 18 (32.7) |
| Clinical benefit rate (at least minimal response) | 29 (52.7) |
| Minimal response | 10 (18.2) |
| Stable disease | 20 (36.4) |
| Progressive disease | 3 (5.5) |
| Unknown* | 3 (5.5) |
| Very good partial response† | 3 (5.5) |
| . | N = 55 . |
|---|---|
| n (%) . | |
| Overall response (at least partial response) | 19 (34.5) |
| Complete response | 0 |
| Near-complete response | 1 (1.8) |
| Partial response | 18 (32.7) |
| Clinical benefit rate (at least minimal response) | 29 (52.7) |
| Minimal response | 10 (18.2) |
| Stable disease | 20 (36.4) |
| Progressive disease | 3 (5.5) |
| Unknown* | 3 (5.5) |
| Very good partial response† | 3 (5.5) |
Patients without postbaseline assessments.
Exploratory endpoint.
Progression-free survival and overall survival. Kaplan-Meier analyses of (A) progression-free survival and (B) overall survival.
Progression-free survival and overall survival. Kaplan-Meier analyses of (A) progression-free survival and (B) overall survival.
The data were analyzed to determine whether timing of the last bortezomib regimen affected response rates. ORR and CBR of the 27 patients who received bortezomib in the last line of therapy before study entry were 25.9% and 48.1%, respectively (Table 3). For the 28 patients who did not receive bortezomib in the last line of therapy, ORR and CBR were 42.9% and 57.1%, respectively. Thus, there was a trend toward a higher response rate in patients whose prior bortezomib therapy was not their last line of therapy, although the numbers are small. Response rates in patients who progressed on their last bortezomib-containing regimen were compared with those in patients who had progressed within 60 days of their last bortezomib-containing regimen. ORR, CBR, and PFS were 37.5%, 55.0%, and 4.2 months in the 40 patients who progressed on bortezomib, and 26.7%, 46.7%, and 7.6 months in the 15 patients who progressed within 60 days of bortezomib (Table 3). Although no trend in response rate was noted, PFS appeared to be longer in those who had progressed within 60 days of their last bortezomib-containing regimen than in those who had progressed while on their last bortezomib-containing regimen.
Overall response rate, clinical benefit rate, and median progression-free survival by baseline characteristics
| . | n . | ORR, % (95% CI) . | CBR, % (95% CI) . | PFS, months (95% CI) . |
|---|---|---|---|---|
| Disease progressed | ||||
| On BTZ | 40 | 37.5 (22.7-54.2) | 55.0 (38.5-70.7) | 4.2 (2.6-5.8) |
| Within 60 d of BTZ | 15 | 26.7 (7.8-55.1) | 46.7 (21.3-73.4) | 7.6 (6.7-9.7) |
| BTZ in last prior line of therapy | ||||
| Yes | 27 | 25.9 (11.1-46.3) | 48.1 (28.7-68.1) | 4.9 (2.1-7.6) |
| No | 28 | 42.9 (24.5-62.8) | 57.1 (37.2-75.5) | 6.0 (3.9-7.6) |
| DEX in last BTZ-containing regimen | ||||
| Yes | 45 | 26.7 (14.6-41.9) | 46.7 (31.7-62.1) | 4.9 (2.6-6.7) |
| No | 10 | 70.0 (34.8-93.3) | 80.0 (44.4-97.5) | 6.2 (2.6-NR) |
| DEX in last prior line of therapy | ||||
| Yes | 37 | 32.4 (18.0-49.8) | 54.1 (36.9-70.5) | 4.2 (2.6-6.7) |
| No | 18 | 38.9 (17.3-64.3) | 50.0 (26.0-74.0) | 6.5 (2.6-9.7) |
| ISS Staging* | ||||
| Stage 1 | 18 | 61.1 (35.8-82.7) | 83.3 (58.6-96.4) | 6.9 (5.4-12.5) |
| Stage 2 | 23 | 21.7 (7.5-43.7) | 34.8 (16.4-57.3) | 3.0 (2.1-4.2) |
| Stage 3 | 13 | 15.4 (1.9-45.5) | 38.5 (13.9-68.4) | 4.9 (1.4-9.0) |
| . | n . | ORR, % (95% CI) . | CBR, % (95% CI) . | PFS, months (95% CI) . |
|---|---|---|---|---|
| Disease progressed | ||||
| On BTZ | 40 | 37.5 (22.7-54.2) | 55.0 (38.5-70.7) | 4.2 (2.6-5.8) |
| Within 60 d of BTZ | 15 | 26.7 (7.8-55.1) | 46.7 (21.3-73.4) | 7.6 (6.7-9.7) |
| BTZ in last prior line of therapy | ||||
| Yes | 27 | 25.9 (11.1-46.3) | 48.1 (28.7-68.1) | 4.9 (2.1-7.6) |
| No | 28 | 42.9 (24.5-62.8) | 57.1 (37.2-75.5) | 6.0 (3.9-7.6) |
| DEX in last BTZ-containing regimen | ||||
| Yes | 45 | 26.7 (14.6-41.9) | 46.7 (31.7-62.1) | 4.9 (2.6-6.7) |
| No | 10 | 70.0 (34.8-93.3) | 80.0 (44.4-97.5) | 6.2 (2.6-NR) |
| DEX in last prior line of therapy | ||||
| Yes | 37 | 32.4 (18.0-49.8) | 54.1 (36.9-70.5) | 4.2 (2.6-6.7) |
| No | 18 | 38.9 (17.3-64.3) | 50.0 (26.0-74.0) | 6.5 (2.6-9.7) |
| ISS Staging* | ||||
| Stage 1 | 18 | 61.1 (35.8-82.7) | 83.3 (58.6-96.4) | 6.9 (5.4-12.5) |
| Stage 2 | 23 | 21.7 (7.5-43.7) | 34.8 (16.4-57.3) | 3.0 (2.1-4.2) |
| Stage 3 | 13 | 15.4 (1.9-45.5) | 38.5 (13.9-68.4) | 4.9 (1.4-9.0) |
BTZ, bortezomib; CBR, clinical benefit rate; CI, confidence interval; DEX, dexamethasone; ISS, International Staging System; NR, not reached; ORR, overall response rate; PFS, progression-free survival.
One patient did not have baseline β2 microglobulin measurement.
The impact of high-risk cytogenetics, as assessed by fluorescence in situ hybridization or conventional cytogenetics, on response rates was analyzed. For the 14 patients with high-risk cytogenetics—defined as del(17p), t(4;14), or t(14;16)—ORR was 42.9% and CBR was 71.4%. Of the 8 patients who had del(17p), the ORR was 37.5% and the CBR was 87.5%. Although the number of patients is small, it does not appear that high-risk cytogenetics adversely affected response rates.
Safety
The most common AEs of any grade regardless of study drug relationship included diarrhea (70.9%), fatigue (69.1%), thrombocytopenia (65.5%), nausea (60.0%), and anemia (47.3%; Table 4). The most common grade 3/4 AEs included thrombocytopenia (63.6%), diarrhea (20.0%), fatigue (20.0%), anemia (14.5%), neutropenia (14.5%), and pneumonia (14.5%). Fatigue and asthenia were managed with hydration, dose reduction, and supportive care. Serious AEs regardless of study drug relationship were reported in 37 patients (67.3%), with those ≥10% limited to thrombocytopenia (25.5%) and pneumonia (14.5%). No significant cardiac abnormalities were noted in the study.
Adverse events regardless of study drug relationship occurring in ≥5% of patients (grade 3/4) (N = 55)
| . | All grades, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|
| Nonhematologic | |||
| Diarrhea | 39 (70.9) | 10 (18.2) | 1 (1.8) |
| Fatigue | 38 (69.1) | 11 (20.0) | 0 |
| Nausea | 33 (60.0) | 3 (5.5) | 0 |
| Hypokalemia | 12 (21.8) | 2 (3.6) | 2 (3.6) |
| Hypotension | 11 (20.0) | 3 (5.5) | 2 (3.6) |
| Asthenia | 11 (20.0) | 5 (9.1) | 0 |
| Abdominal distention | 11 (20.0) | 4 (7.3) | 0 |
| Pneumonia | 9 (16.4) | 6 (10.9) | 2 (3.6) |
| Dehydration | 9 (16.4) | 2 (3.6) | 1 (1.8) |
| Abdominal pain | 9 (16.4) | 3 (5.5) | 0 |
| Flatulence | 6 (10.9) | 3 (5.5) | 0 |
| Sepsis | 5 (9.1) | 2 (3.6) | 3 (5.5) |
| Syncope | 5 (9.1) | 5 (9.1) | 0 |
| Septic shock | 3 (5.5) | 0 | 3 (5.5) |
| Hypophosphatemia | 3 (5.5) | 3 (5.5) | 0 |
| Hematologic | |||
| Thrombocytopenia | 36 (65.5) | 7 (12.7) | 28 (50.9) |
| Anemia | 26 (47.3) | 8 (14.5) | 0 |
| Neutropenia | 10 (18.2) | 5 (9.1) | 3 (5.5) |
| . | All grades, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|
| Nonhematologic | |||
| Diarrhea | 39 (70.9) | 10 (18.2) | 1 (1.8) |
| Fatigue | 38 (69.1) | 11 (20.0) | 0 |
| Nausea | 33 (60.0) | 3 (5.5) | 0 |
| Hypokalemia | 12 (21.8) | 2 (3.6) | 2 (3.6) |
| Hypotension | 11 (20.0) | 3 (5.5) | 2 (3.6) |
| Asthenia | 11 (20.0) | 5 (9.1) | 0 |
| Abdominal distention | 11 (20.0) | 4 (7.3) | 0 |
| Pneumonia | 9 (16.4) | 6 (10.9) | 2 (3.6) |
| Dehydration | 9 (16.4) | 2 (3.6) | 1 (1.8) |
| Abdominal pain | 9 (16.4) | 3 (5.5) | 0 |
| Flatulence | 6 (10.9) | 3 (5.5) | 0 |
| Sepsis | 5 (9.1) | 2 (3.6) | 3 (5.5) |
| Syncope | 5 (9.1) | 5 (9.1) | 0 |
| Septic shock | 3 (5.5) | 0 | 3 (5.5) |
| Hypophosphatemia | 3 (5.5) | 3 (5.5) | 0 |
| Hematologic | |||
| Thrombocytopenia | 36 (65.5) | 7 (12.7) | 28 (50.9) |
| Anemia | 26 (47.3) | 8 (14.5) | 0 |
| Neutropenia | 10 (18.2) | 5 (9.1) | 3 (5.5) |
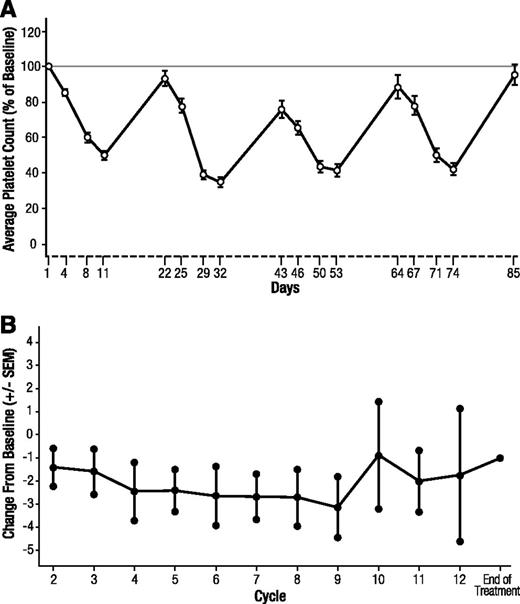
Thrombocytopenia was managed with dose reduction/interruption in 23 patients (41.8%), and 24 patients (43.6%) received ≥1 platelet transfusion (median 2) for the AE. Recovery of platelet levels occurred rapidly during the 1-week treatment-free portion of the schedule (Figure 3A). No patients discontinued because of thrombocytopenia.
Platelet count and neurotoxicity over time. (A) Average platelet count (%) compared with baseline over time. Error bars represent the standard error of the mean (SEM). (B) Change from baseline in the mean Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity subscale. Scales were completed on day 1 of each cycle and at the end of treatment. Error bars represent the SEM.
Platelet count and neurotoxicity over time. (A) Average platelet count (%) compared with baseline over time. Error bars represent the standard error of the mean (SEM). (B) Change from baseline in the mean Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity subscale. Scales were completed on day 1 of each cycle and at the end of treatment. Error bars represent the SEM.
Thirty-eight patients (69.1%) reported grade 1 neuropathy at baseline. Treatment-emergent peripheral neuropathy on this study, 27.3% overall, was generally mild, with only 1 grade 3/4 event (1.8%) reported. Across the study population, the symptoms of peripheral neuropathy, measured by the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity subscale, remained relatively unchanged over the course of the study (Figure 3B).
Discussion
In this phase 2 study, the efficacy of panobinostat in combination with bortezomib and dexamethasone was evaluated in heavily pretreated bortezomib-refractory patients who had received a median of 4 prior regimens, including a median of 2 prior bortezomib-containing regimens. Patients achieved an ORR (≥PR) of 34.5% and a CBR (≥MR) of 52.7%. In a review by Kumar et al, a similar patient population was found to have an ORR to bortezomib-containing therapy of only 20%. Thus, these results compare favorably with currently published data for a refractory MM population.9 ORR of 34.5% in the PANORAMA 2 study exceeded that of 21.8% in patients who achieved ≥PR on the most recent prior regimen, further emphasizing the activity of the combination. Median DoR was 6.0 months and median PFS was 5.4 months. Median OS was not reached after a median follow-up of 8.3 months. These numbers indicate that panobinostat combined with bortezomib and dexamethasone helps to recapture clinical responses in a diverse population of heavily pretreated, bortezomib-refractory patients with MM.
Response rates appeared to be higher in patients whose last bortezomib-containing regimen was not their last line of therapy before study entry. The majority of patients (81.8%) had received dexamethasone with their last bortezomib-based regimen (ie, were refractory to the combination of bortezomib and dexamethasone). A higher response rate observed in the subset of patients who did not have dexamethasone in their last bortezomib-containing regimen could suggest that dexamethasone contributed to the activity in combination with panobinostat and bortezomib; however, the small subset (n = 10) limits more definitive conclusions. It is encouraging that patients with high-risk cytogenetics had similar outcomes to the patient population as a whole, although, again, the patient subgroups were small.
Overall, the combination of panobinostat with bortezomib and dexamethasone was tolerable, with manageable toxicities. Thrombocytopenia was the most common hematologic grade 3/4 AE; however, it did not lead to discontinuation and proved manageable with platelet transfusion and dose reduction/interruption. Thrombocytopenia induced by DACi is a class effect of these agents, where, in a manner similar to that of proteasome inhibitors, panobinostat reversibly blocks maturation of megakaryocytes and the related release of pro-platelets.17-19 In addition, similar to results observed in preclinical studies,17,18 thrombocytopenia rapidly resolved after treatment interruption, with a rebound to near-baseline platelet levels during the treatment-free week of the dosing schedule (Figure 3A).
Peripheral neuropathy is a common toxicity of bortezomib treatment, and 37 patients (67.3%) from this study had reported this AE as part of their prior treatments with bortezomib-based therapy. Most of these prior events were mild (grade 1/2). Similarly, in this trial, on-treatment peripheral neuropathy was mild, with only 1 patient having a grade ≥3 event. These data suggest that panobinostat can be safely combined with bortezomib without significant concern for increasing the severity of peripheral neuropathy.
The findings presented here are consistent with those from the phase 1b trial, which demonstrated efficacy of the panobinostat, bortezomib, and dexamethasone combination in patients with MM, including those refractory to bortezomib therapy.12 The use of another DACi (vorinostat) in MM has been reported previously in a phase 2b study in 143 patients with relapsed and refractory MM. ORR was 11% and DoR was 7.0 months using the EBMT criteria.20 There were key differences between these 2 trials regarding patient eligibility and dexamethasone use. The vorinostat trial included patients with <25% response (ie, stable disease) to prior bortezomib therapy, and dexamethasone could be added only after cycle 4 in patients with no response to the vorinostat combination. These differences limit a direct comparison between the 2 trials.
Other agents in combination with low-dose dexamethasone have shown responses in patients with relapsed and refractory MM. In a randomized trial of pomalidomide and low-dose dexamethasone, the response rate was 34% when pomalidomide was dosed on days 1 to 21 of a 28-day cycle, and 35% when pomalidomide was dosed continuously.21 A phase 2 study of pomalidomide with low-dose dexamethasone in patients with MM resistant to lenalidomide, bortezomib, or both, showed a response rate of 30%.22 In a phase 2 study of carfilzomib and low-dose dexamethasone in patients who had prior therapy with bortezomib and IMiD, an overall response rate of 53% was seen.23 These findings, along with the results of the currently reported trial, provide a rationale for future combination therapy trials using these agents.
The current study points to a role for panobinostat in combination with bortezomib and dexamethasone in the management of bortezomib-refractory patients. In this heavily pretreated population, the combination of panobinostat, bortezomib, and dexamethasone was able to recapture responses in 34.5% of patients. This combination is also being studied in a large phase 3 trial (PANORAMA 1) in relapsed and relapsed and refractory patients with MM.24 Taken together, these trials will better elucidate the role of panobinostat in combination with bortezomib and dexamethasone in advanced relapsed and refractory MM.25
Presented in abstract form at the American Society of Hematology Annual Meeting, Atlanta, GA, December 8-11, 2012.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families as well as the study sites and staff. Peter J. Simon provided editorial assistance to the authors during preparation of this manuscript. The authors also thank Adrian Nöe for critical review of the manuscript.
This study was supported in part by research funding for clinical studies and medical editorial assistance from Novartis Pharmaceuticals Corporation and Novartis research funding (D.M.W.).
Authorship
Contribution: M.A., S.L., S.M., M.S.O., C.S.P., P.G.R., and R.L.S. designed research; M.A., S.E.C., C.G., S.L., S.M., P.G.R., and D.M.W. performed research; S.L., S.M., and P.G.R. contributed vital new reagents or analytical tools; S.E.C., C.G., S.L., S.M., M.S.O., C.S.P., P.G.R., and D.M.W. collected data; M.A., M.K., S.L., S.M., M.S.O., C.S.P., P.G.R., R.L.S., and D.M.W. analyzed and interpreted data; and M.K. and P.G.R. performed statistical analysis. All authors drafted and approved the manuscript.
Conflict-of-interest disclosure: M.A. is a member of the Millennium research funding and advisory board; C.G. is a member of the Millennium and Celgene consultancy and speakers bureaus; P.G.R. is a member of the Bristol-Myers Squibb, Millennium, Johnson & Johnson, and Celgene advisory boards; R.L.S. is a member of the Millennium and Celgene speakers bureaus; M.K., S.M., M.S.O., and C.S.P. are employed by Novartis; and M.K., M.S.O., and C.S.P. own stock in Novartis.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, Harvard University, 450 Brookline Ave, Mailstop Dana 1B02, Boston, MA 02215; e-mail: paul_richardson@dfci.harvard.edu.
References
Author notes
P.G.R. and R.L.S. contributed equally to this study.