Key Points
Ikaros suppresses thymocyte proliferation but induces antiapoptotic molecule expression upon TCR-induced positive selection.
Ikaros function is required to prevent clonal expansion and to maintain a random TCR repertoire during thymocyte differentiation.
Abstract
The zinc-finger protein Ikaros is a key player in T-cell development and a potent tumor suppressor in thymocytes. To understand the molecular basis of its function, we disabled Ikaros activity in vivo using a dominant negative Ikaros transgene (DN-IkTg). In DN-IkTg mice, T-cell development was severely suppressed, and positively selected thymocytes clonally expanded, resulting in a small thymus with a heavily skewed T-cell receptor (TCR) repertoire. Notably, DN-IkTg induced vigorous proliferation concomitant to downregulation of antiapoptotic factor expression such as Bcl2. Ikaros activity was required during positive selection, and specifically at the CD4+CD8lo intermediate stage of thymocyte differentiation, where it prevented persistent TCR signals from inducing aberrant proliferation and expansion. In particular, DN-IkTg induced the accumulation of CD4 single-positive (SP) thymocytes with a developmentally transitional phenotype, and it imposed a developmental arrest accompanied by massive apoptosis. Thus, we identified an in vivo requirement for Ikaros function, which is to suppress the proliferative potential of persistent TCR signals and to promote the survival and differentiation of positively selected thymocytes.
Introduction
Understanding the molecular mechanism of T-cell development is a major question in immunology. T-cell development in the thymus is a highly coordinated process that progresses through distinct developmental stages defined by expression of the coreceptors CD4 and CD8.1 Thymocyte differentiation starts with the most immature thymocytes, which are CD4 and CD8 double-negative (DN), followed by an immature CD4 and CD8 double-positive (DP) stage, and it concludes with lineage choice and differentiation into mature CD4 or CD8 single-positive (SP) cells. These events are primarily driven by T-cell receptor (TCR) signals.2,3 In DN thymocytes, TCR β-selection induces a proliferative burst and their differentiation into DP cells.4 TCR signaling in DP thymocytes, however, does not induce proliferation but induces positive selection and maturation.5,6 Particularly, positive-selecting TCR signals induce termination of Cd8 transcription and differentiation into phenotypically CD4+CD8lo and transcriptionally Cd4+Cd8– intermediate cells.1,7 These intermediate cells are of a transitional phenotype, and they are the immediate precursors of both mature CD4SP and CD8SP thymocytes.8,9 Mature thymocytes regain the ability to proliferate upon TCR stimulation so that DP thymocytes are unique, being refractory to TCR-induced proliferation.
The reason DP thymocytes lose their proliferative potential is unknown. Weak TCR signals induce DP cell differentiation but never induce proliferation. Strong TCR signals, on the other hand, result in programmed cell death but not in cell cycle progression.10 Considering that DP thymocytes are developmentally the first cells to express a clonotypic αβ TCR, it is possible that clonal proliferation in DP cells is suppressed to generate a diverse and balanced TCR repertoire without clonal expansion. If this is true, how is the proliferative arm of TCR signaling suppressed while the differentiation/maturation arm remains intact? To address these questions, we focused our attention on the zinc finger nuclear factor Ikaros as it had been previously proposed as a regulator downstream of TCR signaling to control thymocyte development and CD4/CD8 lineage choice.11,12
Ikaros (Ikzf1) is the prototype of a nuclear zinc-finger protein family that includes Aiolos and Helios in T cells.13-16 Ikaros was originally identified as a T lineage–specific transcription factor involved in TCR regulation, and Ikaros deficiency resulted in impaired T-cell development/activation both in vitro and in vivo.13,17,18 A role of Ikaros downstream of TCR signaling was further reported in Ikaros null mice, which displayed enhanced positive selection, even with reduced TCR signal strength.12,19,20 However, how Ikaros directs TCR signals into distinct developmental outcomes has remained largely unknown. Understanding this process is further complicated because Ikaros undergoes extensive alternative splicing to generate distinct biologically active isoforms. Among these splice variants, isoforms that lack the N-terminal zinc-finger domains, such as Ikaros-7 (Ik7), have been demonstrated to be dominant-negative and to suppress function of all Ikaros family members.11,21,22
Notably, suppression of Ikaros function is highly relevant for leukemogenesis because elevated levels of dominant-negative Ikaros (DN-Ik) isoforms have been found to be associated with acute lymphoblastic leukemia (ALL) in humans23 and with thymoma in mice.24 Thus, in addition to its role in T-cell development and differentiation, Ikaros is also a critical tumor suppressor in lymphoid cells, with an expanding role in other tissues and cells.25
Because DN-Ik is a potent suppressor of Ikaros function, here we used a strategy of overexpressing DN-Ik to assess Ikaros requirement during thymocyte development. We generated mice expressing a T cell–specific dominant-negative Ik7 transgene (DN-IkTg) and found that DN-IkTg induced a dramatic expansion of a unique population of HSAhiTCRβhi CD4SP immature thymocytes. Interestingly, these CD4SP cells proliferated vigorously but did not develop into tumors. Further analysis revealed that such CD4SP cells were TCR-dependent postselection thymocytes, which corresponded to CD4+CD8lo intermediate cells in wild-type (WT) mice. Intermediate cells are positioned at a developmental stage in which TCR signals initiate differentiation and survival but suppress proliferation. In the absence of Ikaros function, however, safeguarding this process was severely impaired, and intermediate cells proliferated in the absence of differentiation. Thus, we identified a novel checkpoint in T-cell development that determines proliferation vs differentiation upon positive selection. These results provide new insights on Ikaros’s function as a tumor suppressor and a driver of T-cell development.
Methods
Mice
C57BL/6 (B6) and MHCIIKO mice were obtained from the Jackson Laboratory. AND TCR transgenic mice26 and human Bcl-2 transgenic mice27 were bred in our own colony. RORγt–EGFP reporter mice28 and HY TCR transgenic Rag2-deficient mice (HY.RAG) were provided by Dr. A. Singer (National Cancer Institute).29 DN-IkTg mice were generated by cloning a murine Ik7 cDNA under the control of human CD2 (hCD2) enhancer-promoter elements and injection into fertilized oocytes. Animal experiments were approved by the National Cancer Institute Animal Care and Use Committee, and all mice were cared for in accordance with National Institutes of Health guidelines.
Intracellular staining and flow cytometry
Cells were harvested, stained, and analyzed on a FACS LSRII or FACS AriaII. Dead cells were excluded by forward light scatter gating and propidium iodide staining. Data were analyzed using software designed by the Division of Computer Research and Technology at the National Institutes of Health. Antibodies with the following specificities were used for staining: TCRβ, CD3ε, CD4, CD8α, CD25, CCR7 (all from BD Biosciences); Qa-2, Ki-67, interleukin (IL)-2Rβ, HSA, IL-7Rα, CD44, CD62L, CD69, interferon (IFN)γ, and IL-4 (all from eBioscience). For intracellular cytokine staining, cells were restimulated for 3 hours with phorbol 12-myristate 13-acetate and ionomycin with the addition of brefeldin A, then fixed and permeabilized with intracellular fixation and permeabilization buffer (eBioscience). TCR Vβ staining was carried out using an anti-Vβ panel antibody kit (BD Biosciences). Intracellular Bcl2 expression was assessed with an anti-mouse Bcl2 staining kit (BD Biosciences). For DN thymocyte analysis, CD8+-depleted thymocytes were incubated with the following biotinylated antibodies: anti-TCRβ, -B220, -CD8β, -GL3, -DX5, -MAC1, and -GR1, followed by FITC-conjugated streptavidin. FITC signal–negative thymocytes were then assessed for CD44 and CD25 expression using PE-conjugated anti-CD44 and APC-conjugated anti-CD25 antibodies (all from BD Biosciences).
Cell isolation and in vitro cell culture
Naïve CD4+ T cells were electronically sorted using anti-CD4, CD8α, CD62L, CD44, and CD25 antibodies, gated on CD62L+CD44loCD25– cells. CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (all from eBioscience) and cultured under Th0 conditions (medium), or they were differentiated into Th1 cells by culture for 5 days with mouse IL-12 (10 ng/mL; Peprotech) and anti-mouse IL-4 antibodies (10 μg/mL; eBioscience) or into Th2 cells by culture for 5 days with mouse IL-4 (10 ng/mL; Peprotech) and anti-IFNγ antibodies (10 μg/mL; eBioscience). IL-4 and IFNγ concentrations in culture supernatants were analyzed and quantitated by enzyme-linked immunosorbent assay (R&D Systems).
Immunoblotting
Lymph nodes (LN) and thymocytes were lysed in Cell Lytic-M (Sigma). Whole-cell lysates were electrophoresed on 4% to 12% Bis-Tris gels and transferred to nitrocellulose membranes (Invitrogen). Blots were incubated with anti–N-terminal Ikaros (Abcam) or anti–C-terminal Ikaros (Santa Cruz Biotechnology) antibodies and visualized using horseradish peroxidase–conjugated anti-rabbit IgG antibodies. Reactivity was visualized by enhanced chemiluminescence (Pierce).
BrdU cell proliferation assay
Cell proliferation was measured by BrdU (5-bromodeoxyuridine) incorporation. B6 or DN-IkTg mice were given intraperitoneal injections of BrdU dissolved in phosphate-buffered saline (1 mg/mouse) and analyzed 1 day later. Thymocytes were first stained with anti-CD4, anti-TCRβ, anti-CD8α, and anti-HSA, and then fixed and permeabilized with Cytofix/Cytoperm solution and Cytofix/Cytoperm Plus for intranuclear anti-BrdU staining according to the manufacturer’s protocol (BD Biosciences).
Quantitative real-time PCR
LN T cells were isolated by depleting B cells with anti-mouse IgG beads, and CD4+ and CD8+ LN T cells were further purified by depleting them with either anti-CD8 mAbs or anti-CD4 mAbs. Total RNA was isolated with the RNeasy kit (Qiagen). RNA was reverse-transcribed into cDNA by oligo(dT) priming with the QuantiTect reverse transcription kit (Qiagen). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with an ABI PRISM 7900HT Sequence Detection System and the QuantiTect SYBR Green detection system (Qiagen).
Gene array analysis
RNA was isolated with the RNeasy kit (Qiagen) from electronically sorted CD4SP thymocytes. RNA was reverse-transcribed into cDNA by oligo(dT) priming with the QuantiTect reverse transcription kit (Qiagen). The expression of 84 key genes involved in apoptosis, or programmed cell death, was performed with the mouse apoptosis RT2Profiler™ PCR array system (PAMM-012 E; SABiosciences). PCR array data were analyzed by the ΔΔCt data analysis method.
Microscopy
Microscopy was done on a Zeiss Axio Observer Z1 microscope using a 10× Plan-Apochromat (numerical aperture 0.45) objective lens at room temperature (Carl Zeiss Microscopy, LLC, Thornwood, NY). Images were captured with a Zeiss AxioCam MRc5 color CCD camera (Carl Zeiss Microscopy). Zeiss AxioVision software (version 4.8) was used to acquire an array of tile images that covered the entire area of the sample, and those were stitched together to form a single large image. Adobe Photoshop (version CS4) was used to adjust the brightness and contrast by linear histogram stretching and arrange the images into figures.
Statistical analysis
All statistical tests were done using PRISM GraphPad software. Statistical significance was determined by Student t test. P < .05 was considered significant.
Results
DN Ikaros impairs T-cell development in the thymus
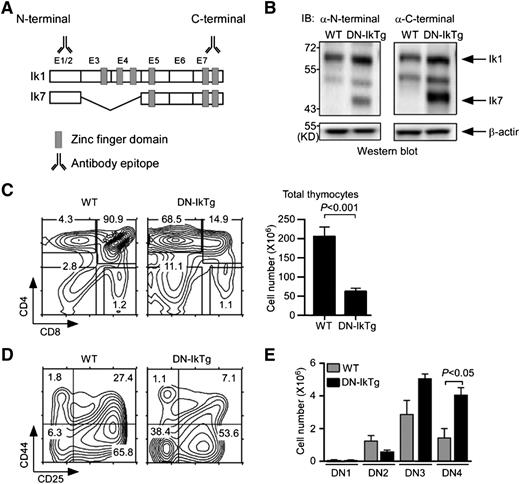
Thymocytes express a number of Ikaros family molecules including Ikaros, Aiolos, Helios, and Pegasus.13,16,17,30,31 To suppress their function during T-cell development, we expressed a DN Ikaros isoform in thymocytes. Specifically, we generated transgenic mice expressing Ik7 under control of the hCD2 enhancer/promoter. Ik7 is an Ikaros splice isoform that lacks exons 3 and 4 of the full-length Ikaros-1 (Figure 1A). Zinc fingers in Ikaros exons 3 and 4 are necessary for DNA binding.11,15 Consequently, Ik7 acts as a DN form of Ikaros by dimerizing with intact Ikaros family molecules and preventing their binding to DNA.21 To confirm transgenic DN-IkTg expression, we first assessed Ikaros expression in WT and DN-IkTg thymocytes by immunoblot analysis (Figure 1B). Both WT and DN-IkTg thymocytes expressed full-length Ikaros proteins (Ik1), but only DN-IkTg thymocytes expressed Ik7 as determined using Ikaros N-terminal and C-terminal epitope-specific antibodies (Figure 1B). We also confirmed Ik7 expression in DN-IkTg LN T cells (supplemental Figure 1A), which were reduced in total numbers compared with WT mice (supplemental Figure 1B-C). Importantly, DN-IkTg CD4+ T cells showed a heavily skewed Th1 phenotype (supplemental Figure 1D), which is in agreement with previous reports on DN-Ikaros function in T cells.32,33 These results confirm successful expression of a functional DN-IkTg in thymocytes and T cells.
Dominant-negative Ikaros impairs T-cell development in the thymus. (A) Exon organization of full-length Ikaros-1 (Ik1) and the DN splice variant Ik7. E1-E7 corresponds to exon 1-exon 7. Antibody binding sites indicate epitopes of immunoblot antibodies. (B) Immunoblot analysis of WT and DN-IkTg thymocytes. Whole-cell lysates were probed with Ikaros N-terminal (left) or C-terminal (right)–specific antibodies. The same blot was reprobed with anti–β-actin antibodies for loading control. The blots are representative of 3 independent experiments with each 1 WT and 1 DN-IkTg mice. (C) Thymocyte profiles and cell numbers of WT and DN-IkTg mice. Contour plots show CD4/CD8 profiles of total thymocytes (left). The bar graphs indicate the mean ± SEM of total thymocyte numbers (right). Data represent a summary of 11 WT and 13 DN-IkTg mice from 11 independent experiments. (D) CD44 vs CD25 expression in lineage marker–negative, immature DN thymocytes. Contour plots are representative of 4 independent experiments with each 1 WT and 1 DN-IkTg mouse. (E) Cell numbers of individual DN subpopulations. The bar graphs indicate the mean ± SEM of 3 independent experiments with each 1 WT and 1 DN-IkTg mouse.
Dominant-negative Ikaros impairs T-cell development in the thymus. (A) Exon organization of full-length Ikaros-1 (Ik1) and the DN splice variant Ik7. E1-E7 corresponds to exon 1-exon 7. Antibody binding sites indicate epitopes of immunoblot antibodies. (B) Immunoblot analysis of WT and DN-IkTg thymocytes. Whole-cell lysates were probed with Ikaros N-terminal (left) or C-terminal (right)–specific antibodies. The same blot was reprobed with anti–β-actin antibodies for loading control. The blots are representative of 3 independent experiments with each 1 WT and 1 DN-IkTg mice. (C) Thymocyte profiles and cell numbers of WT and DN-IkTg mice. Contour plots show CD4/CD8 profiles of total thymocytes (left). The bar graphs indicate the mean ± SEM of total thymocyte numbers (right). Data represent a summary of 11 WT and 13 DN-IkTg mice from 11 independent experiments. (D) CD44 vs CD25 expression in lineage marker–negative, immature DN thymocytes. Contour plots are representative of 4 independent experiments with each 1 WT and 1 DN-IkTg mouse. (E) Cell numbers of individual DN subpopulations. The bar graphs indicate the mean ± SEM of 3 independent experiments with each 1 WT and 1 DN-IkTg mouse.
To understand the role of Ikaros in T-cell development, we next examined CD4 and CD8 coreceptor expression on DN-IkTg thymocytes. Strikingly, we found a dramatic accumulation of CD4 SP cells and a significant decrease in overall thymocyte numbers (Figure 1C). Reduced thymocyte numbers were likely the result of a developmental block at the CD4 and CD8 DN stage, because immature DN thymocyte percentages were dramatically increased (Figure 1C, left). Moreover, DN cell differentiation was blocked at the DN3/DN4 stages (Figure 1D), resulting in increased cell numbers in these compartments (Figure 1E). These data document a requirement for Ikaros in promoting early DN thymocyte differentiation.
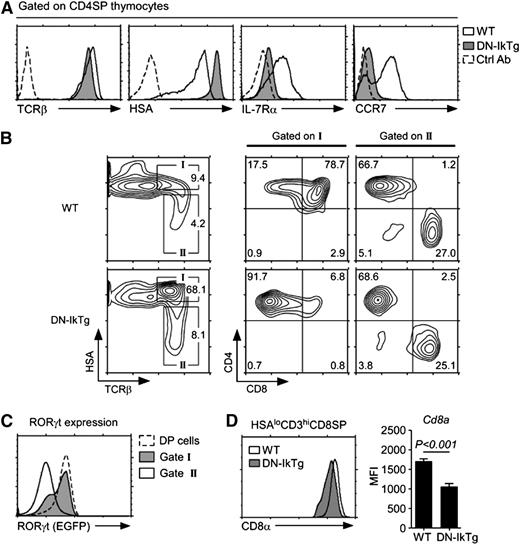
Unlike immature DN thymocytes, CD4SP thymocytes are mature, end-differentiated cells and their lineage fates have been sealed.34,35 CD4SP thymocytes in DN-IkTg mice, however, displayed a mixed phenotype of mature and immature cell markers (Figure 2A). Specifically, DN-IkTg CD4SP cells expressed high levels of TCRβ, like mature thymocytes, but they also expressed high levels of HSA and low levels of IL-7Rα and CCR7, much like immature thymocytes (Figure 2A). Thus, DN-IkTg CD4SP cells displayed a transitional phenotype that is reminiscent of intermediate thymocytes.9 Intermediate cells are the progeny of TCR-signaled DP thymocytes, which terminate CD8 transcription to become transcriptionally Cd4+Cd8– and phenotypically CD4+CD8lo cells.7,36 Developmentally, these cells are positioned at the HSAhiTCRβhi stage, as indicated by “gate I” in an HSA vs TCRβ plot (Figure 2B). In comparison, fully mature thymocytes are phenotypically HSAloTCRβhi and are found in “gate II.”37 Interestingly, DN-IkTg expression induced a dramatic accumulation of CD4SP semimature cells (gate I), which we think correspond to CD4+CD8lo intermediate cells in WT thymocytes. To assess whether DN-IkTg CD4SP cells are developmentally immature despite expressing high levels of TCRβ, we examined RORγt expression in vivo using RORγt-EGFP reporter mice. RORγt is a transcription factor highly expressed in immature DP cells and completely absent in mature thymocytes.28 In DN-IkTg mice expressing the RORγt-EGFP reporter, we found that CD4SP cells still expressed high levels of RORγt (Figure 2C), demonstrating that these cells remain immature and are developmentally arrested at this stage.
Accumulation of CD4SP cells with a developmentally intermediate phenotype in DN-IkTg thymocytes. (A) Maturation marker expression on CD4SP cells from WT and DN-IkTg thymocytes. Cell surface expression of indicated surface proteins were assessed for WT (open histogram) and DN-IkTg (shaded histogram) cells and overlaid with control Ab staining (dotted line). Data are representative of 13 independent experiments with at least 10 WT and 13 DN-IkTg mice. (B) Developmental arrest in DN-IkTg thymocytes. Contour plots show HSA vs surface TCRβ expression on total thymocytes (left). Box I identifies HSAhiTCRβhi immature thymocytes, which underwent positive selection. Box II indicates HSAloTCRβhi mature postselection thymocytes. CD4 vs CD8 profiles of box I or box II gated thymocytes are shown on the right. Contour plots are representative of 8 independent experiments that analyzed 10 WT and 13 DN-IkTg mice. (C) RORγt expression in RORγt-EGFP.DN-IkTg mouse thymocytes. RORγt transcription (EGFP expression) was assessed in the indicated cell populations of DN-IkTg mice expressing an RORγt-EGFP reporter allele. The histogram is representative of 3 independent experiments with 5 RORγt-EGFP and 9 RORγt-EGFP.DN-IkTg mice. (D) Surface CD8α levels on mature HSAloCD3hi CD8SP thymocytes. The histogram is representative, and the bar graph shows the mean ± SEM, of 7 independent experiments with each 1 WT and 1 DN-IkTg mouse.
Accumulation of CD4SP cells with a developmentally intermediate phenotype in DN-IkTg thymocytes. (A) Maturation marker expression on CD4SP cells from WT and DN-IkTg thymocytes. Cell surface expression of indicated surface proteins were assessed for WT (open histogram) and DN-IkTg (shaded histogram) cells and overlaid with control Ab staining (dotted line). Data are representative of 13 independent experiments with at least 10 WT and 13 DN-IkTg mice. (B) Developmental arrest in DN-IkTg thymocytes. Contour plots show HSA vs surface TCRβ expression on total thymocytes (left). Box I identifies HSAhiTCRβhi immature thymocytes, which underwent positive selection. Box II indicates HSAloTCRβhi mature postselection thymocytes. CD4 vs CD8 profiles of box I or box II gated thymocytes are shown on the right. Contour plots are representative of 8 independent experiments that analyzed 10 WT and 13 DN-IkTg mice. (C) RORγt expression in RORγt-EGFP.DN-IkTg mouse thymocytes. RORγt transcription (EGFP expression) was assessed in the indicated cell populations of DN-IkTg mice expressing an RORγt-EGFP reporter allele. The histogram is representative of 3 independent experiments with 5 RORγt-EGFP and 9 RORγt-EGFP.DN-IkTg mice. (D) Surface CD8α levels on mature HSAloCD3hi CD8SP thymocytes. The histogram is representative, and the bar graph shows the mean ± SEM, of 7 independent experiments with each 1 WT and 1 DN-IkTg mouse.
Conversely, some thymocytes escaped such developmental arrest and they fully differentiated into HSAlo mature thymocytes (Figure 2B, gate II). Notably, the CD4/CD8 ratio in gate II mature thymocytes was comparable with WT mice, suggesting that DN-IkTg did not affect lineage choice. The resulting CD8SP cells, however, showed significantly reduced levels of CD8 coreceptor expression (Figure 2D). Ikaros is a critical regulator of CD8α transcription,38 and these data suggest that DN-Ikaros interferes with CD8α expression in vivo and potentially with CD8+ T-cell function.29 Taken together, these results reveal a new developmental check point in CD4+CD8lo intermediate cells that is dependent on Ikaros function.
Aberrant proliferation and accumulation of CD4SP thymocytes in DN-IkTg mice
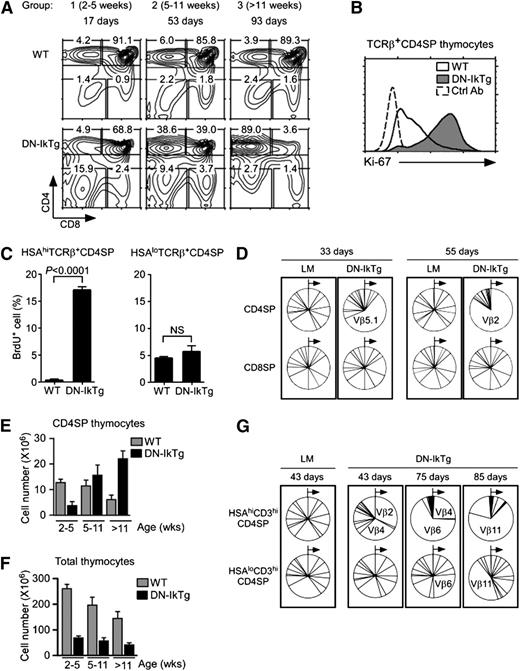
To further investigate the DN-IkTg effect on T-cell development, we analyzed the thymi of newborn DN-IkTg mice and tracked their thymocyte profiles with advancing age. Mice were placed into 3 groups based on age (Figure 3A): group 1 (2-5 weeks), group 2 (5-11 weeks), and group 3 (>11 weeks). Already in very young mice (2-5 weeks), we found a profound decrease in total thymocyte numbers (supplemental Figure 2A). However, CD4SP frequencies were comparable with WT thymocytes, and we did not observe preferential accumulation of CD4SP cells (Figure 3A). But then, TCRβ+ CD4SP frequencies in DN-IkTg mice increased rapidly and dramatically with age, ultimately occupying the vast majority of thymocytes in older mice (Figure 3A). These data suggest that the increased proportion of DN-IkTg CD4SP thymocytes is caused by CD4SP cell accumulation with age and not because of skewed CD4 lineage commitment during thymocyte differentiation.
Clonal expansion of CD4SP cells in DN-IkTg thymocytes. (A) Thymocyte profiles of DN-IkTg mice with progressing age. Contour plots show representative CD4/CD8 thymocyte profiles of WT and DN-IkTg mice at different ages. Data are representative of at least 5 independent experiments with 21 WT and 27 DN-IkTg mice. (B) Intracellular Ki-67 staining in TCRβ+CD4SP thymocytes. Data are representative of 3 independent experiments with 4 WT and 6 DN-IkTg mice. The histogram shows data from 49-day-old WT and 80-day-old DN-IkTg mouse. (C) BrdU labeling assays of WT and DN-IkTg thymocytes. BrdU incorporation was determined in immature (HSAhiTCRβ+) and mature (HSAloTCRβ+) CD4SP thymocytes by intracellular staining. Data are the summary of 2 independent experiments with 3 WT mice (two 55 days old and one 58 days old) and 4 DN-IkTg mice (45, 62, 62, and 82 days old). (D) TCR Vβ distribution in CD4SP and CD8SP thymocytes. LM and DN-IkTg thymocytes were stained for a panel of 15 different TCR Vβ segments starting from Vβ2 through Vβ17a in a clockwise direction (see Methods). Relative expression of individual TCR Vβ on CD4SP and CD8SP cells are shown in pie charts for each individual mouse. Each pie chart represents an independent experiment. Data show the representative results from 2 independent experiments with each 1 age-matched (33 and 55 days old) WT and DN-IkTg mouse, respectively. (E) CD4SP thymocyte numbers in WT and DN-IkTg mice with progressing age. CD4SP cell numbers were determined in 3 different age groups of WT and DN-IkTg mice: group 1 (2-5 weeks), group 2 (5-11 weeks), and group 3 (>11 weeks). Data represent the summary of at least 4 independent experiments with 17 WT and 23 DN-IkTg mice. Bar graphs represent mean ± SEM. (F) Total thymocyte numbers of WT and DN-IkTg mice with progressing age. Thymocyte numbers were determined in the indicated age groups with 21 WT and 27 DN-IkTg mice. Data show the summary of at least 5 independent experiments and represent the mean ± SEM. (G) TCR Vβ distribution in HSAhi and HSAloCD3hiCD4SP thymocytes. Indicated thymocyte populations from LM and DN-IkTg mice were stained for a panel of 15 different TCR Vβ segments whose relative distributions are shown in pie charts. Each box indicates analyses from a single mouse.
Clonal expansion of CD4SP cells in DN-IkTg thymocytes. (A) Thymocyte profiles of DN-IkTg mice with progressing age. Contour plots show representative CD4/CD8 thymocyte profiles of WT and DN-IkTg mice at different ages. Data are representative of at least 5 independent experiments with 21 WT and 27 DN-IkTg mice. (B) Intracellular Ki-67 staining in TCRβ+CD4SP thymocytes. Data are representative of 3 independent experiments with 4 WT and 6 DN-IkTg mice. The histogram shows data from 49-day-old WT and 80-day-old DN-IkTg mouse. (C) BrdU labeling assays of WT and DN-IkTg thymocytes. BrdU incorporation was determined in immature (HSAhiTCRβ+) and mature (HSAloTCRβ+) CD4SP thymocytes by intracellular staining. Data are the summary of 2 independent experiments with 3 WT mice (two 55 days old and one 58 days old) and 4 DN-IkTg mice (45, 62, 62, and 82 days old). (D) TCR Vβ distribution in CD4SP and CD8SP thymocytes. LM and DN-IkTg thymocytes were stained for a panel of 15 different TCR Vβ segments starting from Vβ2 through Vβ17a in a clockwise direction (see Methods). Relative expression of individual TCR Vβ on CD4SP and CD8SP cells are shown in pie charts for each individual mouse. Each pie chart represents an independent experiment. Data show the representative results from 2 independent experiments with each 1 age-matched (33 and 55 days old) WT and DN-IkTg mouse, respectively. (E) CD4SP thymocyte numbers in WT and DN-IkTg mice with progressing age. CD4SP cell numbers were determined in 3 different age groups of WT and DN-IkTg mice: group 1 (2-5 weeks), group 2 (5-11 weeks), and group 3 (>11 weeks). Data represent the summary of at least 4 independent experiments with 17 WT and 23 DN-IkTg mice. Bar graphs represent mean ± SEM. (F) Total thymocyte numbers of WT and DN-IkTg mice with progressing age. Thymocyte numbers were determined in the indicated age groups with 21 WT and 27 DN-IkTg mice. Data show the summary of at least 5 independent experiments and represent the mean ± SEM. (G) TCR Vβ distribution in HSAhi and HSAloCD3hiCD4SP thymocytes. Indicated thymocyte populations from LM and DN-IkTg mice were stained for a panel of 15 different TCR Vβ segments whose relative distributions are shown in pie charts. Each box indicates analyses from a single mouse.
Because of such rapid accumulation, next we wished to know whether DN-IkTg CD4SP cells were proliferating. Upon Ki-67 expression analysis, we found that most DN-IkTg CD4SP cells expressed high levels of this cell proliferation–associated nuclear antigen (Figure 3B). CD8SP cells, on the other hand, were Ki-67–negative in both DN-IkTg and WT mice (supplemental Figure 2B). To confirm active cell proliferation in vivo, we next examined BrdU incorporation in HSAhi and HSAloTCRβhi CD4SP thymocytes (Figure 3C). Semimature HSAhiTCRβhi CD4SP cells in DN-IkTg mice contained high percentages of BrdU+ cells (>15%), whereas the same population in WT mice was virtually void of BrdU+ cells (<0.05%). Interestingly, such differences in BrdU levels were not observed in mature HSAloTCRβhi CD4SP cells (Figure 3C, right). Thus, Ikaros activity is required to suppress proliferation specifically in HSAhiTCRβhi CD4SP thymocytes. DN-Ikaros, on the other hand, permits uncontrolled proliferation of intermediate phenotype CD4SP cells.
To test whether such proliferation would result in the expansion of an oligoclonal cell population, we assessed the TCR Vβ repertoire and found that TCRβ chain usage of DN-IkTg CD4SP cells were dominated by random TCR Vβ clones (Figure 3D, top). Such clonal expansion became apparent around 4 weeks of age (supplemental Figure 2C), and it was specific to CD4SP thymocytes because TCR Vβ expression in CD8SP cells (Figure 3D, bottom) and in peripheral CD4 and CD8 LN T cells remained largely unaffected (supplemental Figure 2D). Also such clonal expansion was only manifested in postselection thymocytes because we did not find skewed TCR Vβ repertoires in CD69+ thymocytes that underwent positive selection (supplemental Figure 2E). Thus, DN-Ikaros induced the accumulation of a mono- or oligoclonal CD4SP thymocyte population whose absolute cell numbers increased with age (Figure 3E) but without increasing overall thymocyte numbers at the same time (Figure 3F). Importantly, although CD4SP accumulation was largely restricted to the HSAhi population, some of the clonally expanded cells escaped and matured into HSAlo CD4SP cells, partially skewing the mature thymocyte repertoire (Figure 3G). Collectively, these results suggest that DN-Ikaros induces a developmental arrest at the HSAhiTCRβ+ semimature stage and they reveal a role for Ikaros in maintaining a diverse and balanced TCR repertoire in developing thymocytes.
DN-IkTg induces programmed cell death in CD4SP thymocytes
Both the oligoclonal origin and the rapid proliferation of DN-IkTg CD4SP cells suggested the possibility of thymoma formation. However, DN-IkTg thymocyte numbers actually decreased with age (Figure 3F), and DN-IkTg mice never developed tumors (supplemental Table 1). Consequently, we wished to know what prevented DN-IkTg CD4SP cells from developing into tumors.
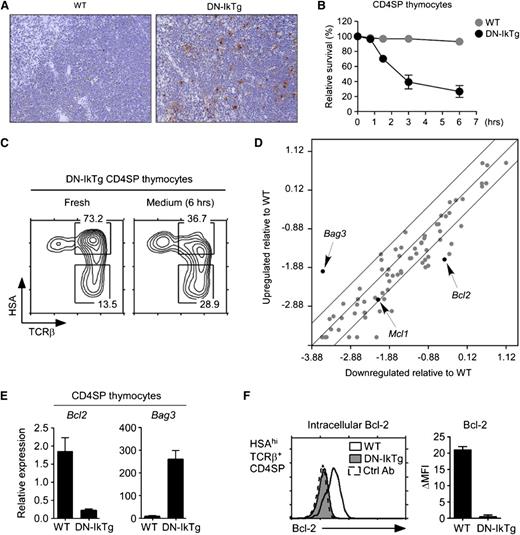
Notably, we found dramatically increased numbers of apoptotic cells in DN-IkTg mice by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays (Figure 4A). To identify which cells were undergoing apoptosis, we cultured thymocytes in vitro and determined the kinetics of cell death in individual populations. Although cell viabilities of freshly isolated DN-IkTg CD4SP and WT CD4SP thymocytes were comparable, DN-IkTg CD4SP cells underwent rapid cell death in vitro (Figure 4B; supplemental Figure 3A). As a result, after 6 hours’ culturing in medium, the majority of HSAhiTCRβ+ cells disappeared and the surviving CD4SP thymocytes were enriched for HSAloTCRβ+ cells (Figure 4C). This was specific to DN-IkTg CD4SP cells because WT CD4SP or DN-IkTg CD8SP cells maintained cell viability under both short-term and O/N in vitro culture conditions (supplemental Figure 3).
Massive apoptosis of DN-IkTg CD4SP thymocytes. (A) TUNEL assay of WT and DN-IkTg thymus sections. Upon TUNEL staining, thymus tissue sections were counterstained with hematoxylin and eosin. Histology slides show results from a 93-day-old WT and a 94-day-old DN-IkTg mouse and are representative of 3 independent experiments (original magnification ×10) for a total of 3 WT and 3 DN-IkTg mice. (B) Survival kinetics of DN-IkTg CD4SP thymocytes in vitro. Cell viabilities were determined by propidium iodide exclusion at indicated time points during in vitro culture. Data show the summary of 3 independent experiments with each 1 WT and 2 DN-IkTg mice, respectively. (C) Phenotype analysis of apoptosis-resistant DN-IkTg CD4SP cells. Freshly isolated DN-IkTg thymocytes were incubated in medium for 6 hours and then assessed for cell survival. Propidium iodide staining–negative cells were analyzed for their CD4/CD8 profiles and HSA/TCRβ expression. Contour plots are representative of 2 independent experiments with 3 WT and 4 DN-IkTg mice. (D) Apoptosis pathway gene array analysis of DN-IkTg CD4SP thymocytes. Total RNA from sorted WT or DN-IkTg CD4SP cells were used to probe an apoptosis PCR array for expression of pro- and antiapoptotic genes. Data are representative of 3 independent experiments using sorted CD4SP thymocytes with each 1 WT and 1 DN-IkTg mouse. (E) qRT-PCR analysis of potential Ikaros target gene expression from sorted CD4SP thymocytes. Data are the mean ± SEM of 3 independent experiments with a total of 3 WT and 4 DN-IkTg mice. (F) Intracellular Bcl-2 expression in HSAhiCD3hiCD4SP. Freshly isolated DN-IkTg thymocytes were assessed for Bcl-2 expression. The histogram is representative of, and the bar graph displays, the mean ± SEM of 2 independent experiments with each 1 WT and 2 DN-IkTg mouse.
Massive apoptosis of DN-IkTg CD4SP thymocytes. (A) TUNEL assay of WT and DN-IkTg thymus sections. Upon TUNEL staining, thymus tissue sections were counterstained with hematoxylin and eosin. Histology slides show results from a 93-day-old WT and a 94-day-old DN-IkTg mouse and are representative of 3 independent experiments (original magnification ×10) for a total of 3 WT and 3 DN-IkTg mice. (B) Survival kinetics of DN-IkTg CD4SP thymocytes in vitro. Cell viabilities were determined by propidium iodide exclusion at indicated time points during in vitro culture. Data show the summary of 3 independent experiments with each 1 WT and 2 DN-IkTg mice, respectively. (C) Phenotype analysis of apoptosis-resistant DN-IkTg CD4SP cells. Freshly isolated DN-IkTg thymocytes were incubated in medium for 6 hours and then assessed for cell survival. Propidium iodide staining–negative cells were analyzed for their CD4/CD8 profiles and HSA/TCRβ expression. Contour plots are representative of 2 independent experiments with 3 WT and 4 DN-IkTg mice. (D) Apoptosis pathway gene array analysis of DN-IkTg CD4SP thymocytes. Total RNA from sorted WT or DN-IkTg CD4SP cells were used to probe an apoptosis PCR array for expression of pro- and antiapoptotic genes. Data are representative of 3 independent experiments using sorted CD4SP thymocytes with each 1 WT and 1 DN-IkTg mouse. (E) qRT-PCR analysis of potential Ikaros target gene expression from sorted CD4SP thymocytes. Data are the mean ± SEM of 3 independent experiments with a total of 3 WT and 4 DN-IkTg mice. (F) Intracellular Bcl-2 expression in HSAhiCD3hiCD4SP. Freshly isolated DN-IkTg thymocytes were assessed for Bcl-2 expression. The histogram is representative of, and the bar graph displays, the mean ± SEM of 2 independent experiments with each 1 WT and 2 DN-IkTg mouse.
To understand the cell death mechanism downstream of DN-IkTg, we performed mRNA microarray analyses with purified CD4SP thymocytes from DN-IkTg and WT mice (Figure 4D). Specifically, we screened for genes involved in cell survival and apoptosis and found that expression of most antiapoptotic genes was downregulated in DN-IkTg CD4SP thymocytes (supplemental Table 2). We obtained similar results when performing gene array analysis on sorted HSAhiTCRβ+CD4SP thymocytes (supplemental Figure 4A, left). In mature HSAloCD4SP thymocytes, however, gene expression was more homogenous between WT and DN-IkTg mice (supplemental Figure 4A, right), which can be explained by reduced DN-Ikaros transgene expression in these cells (supplemental Figure 4B).
Finally, the microarray results were confirmed by quantitative real-time PCR for Bcl2 and Bag3 gene expression (microarray GEO accession numbers: GSE49264, GSE49270, GSE49271) and also by intracellular staining (Figure 4E-F). Although the role of Bag3 in cell death remains unknown,39 downregulation of the antiapoptotic Bcl2 in DN-IkTg CD4SP cells suggested that Ikaros function is necessary for survival of proliferating CD4SP cells. Collectively, these data suggest that DN-IkTg CD4SP cells fail to induce thymoma formation, presumably because of increased susceptibility to cell death.
Bcl2 prevents cell death but fails to induce tumor in DN-IkTg thymocytes
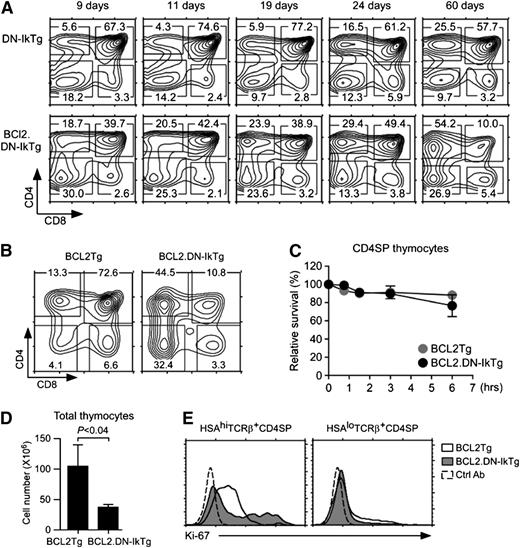
To test whether preventing apoptosis would induce tumor of proliferating DN-IkTg CD4SP cells, we next crossed DN-IkTg mice with Bcl2 transgenic mice (BCL2.DN-IkTg). In BCL2.DN-IkTg mice, we found that CD4SP cells accumulated with much faster kinetics than in control DN-IkTg mice, suggesting that apoptosis plays a central role in the accumulation of these cells (Figure 5A). Nevertheless, the thymic profile remained stable in aged BCL2.DN-IkTg mice, displaying a dominant CD4SP and a significantly increased DN cell population compared with littermate (LM) Bcl2 transgenic thymocytes (Figure 5B). Importantly, we never observed thymic lymphoma in these mice despite improved cell viability of CD4SP cells (Figure 5C; supplemental Figure 5A-B). In fact, overall thymocyte numbers were decreased compared with Bcl2Tg control thymuses (Figure 5D). To understand why survival was insufficient to induce tumor of proliferating CD4SP cells, we next assessed intracellular Ki-67 expression. Surprisingly, unlike DN-IkTg CD4SP cells (Figure 3B), Bcl2 transgenic DN-IkTg CD4SP cells did not express Ki-67, indicating that they have become nonproliferating quiescent cells (Figure 5E). These results are in agreement with previous studies, where transgenic Bcl2 reportedly suppressed cell cycle progression.40 Thus, programmed cell death of DN-IkTg CD4SP cells can be rescued by Bcl2, but DN-IkTg still fails to induce tumor because Bcl2 now suppressed proliferation. In summary, DN Ikaros permits uncontrolled cell proliferation, but without concomitant induction of survival factors, so tumors fail to develop.
Transgenic Bcl-2 rescues programmed cell death but fails to restore T-cell development in DN-IkTg mice. (A) Thymocyte profiles of BCL2.DN-IkTg mice with progressing age. Contour plots show CD4/CD8 profiles of BCL2.DN-IkTg and control DN-IkTg thymocytes at the indicated age. (B) Thymocyte profiles of BCL2Tg and BCL2.DN-IkTg mice. Contour plots show CD4 vs CD8 profiles of total thymocytes. Contour plots are from age-matched mice (81 days old) and are representative of 4 independent experiments with 4 BCL2Tg and 7 BCL2.DN-IkTg mice. (C) Survival kinetics of BCL2.DN-IkTg CD4SP thymocytes in vitro. Cell viabilities were determined by propidium iodide exclusion at the indicated time points. Data show the summary of 2 independent experiments with 2 BCL2Tg and 3 BCL2.DN-IkTg mice. (D) Total thymocyte numbers of BCL2Tg and BCL2.DN-IkTg mice. Bar graphs show the mean ± SEM of at least 4 independent experiments with 4 BCL2Tg and 7 BCL2Tg.DN-IkTg mice. (E) Intracellular Ki-67 staining in HSAhi and HSAloTCRβ+ CD4SP thymocytes. Data are representative of 4 independent experiments.
Transgenic Bcl-2 rescues programmed cell death but fails to restore T-cell development in DN-IkTg mice. (A) Thymocyte profiles of BCL2.DN-IkTg mice with progressing age. Contour plots show CD4/CD8 profiles of BCL2.DN-IkTg and control DN-IkTg thymocytes at the indicated age. (B) Thymocyte profiles of BCL2Tg and BCL2.DN-IkTg mice. Contour plots show CD4 vs CD8 profiles of total thymocytes. Contour plots are from age-matched mice (81 days old) and are representative of 4 independent experiments with 4 BCL2Tg and 7 BCL2.DN-IkTg mice. (C) Survival kinetics of BCL2.DN-IkTg CD4SP thymocytes in vitro. Cell viabilities were determined by propidium iodide exclusion at the indicated time points. Data show the summary of 2 independent experiments with 2 BCL2Tg and 3 BCL2.DN-IkTg mice. (D) Total thymocyte numbers of BCL2Tg and BCL2.DN-IkTg mice. Bar graphs show the mean ± SEM of at least 4 independent experiments with 4 BCL2Tg and 7 BCL2Tg.DN-IkTg mice. (E) Intracellular Ki-67 staining in HSAhi and HSAloTCRβ+ CD4SP thymocytes. Data are representative of 4 independent experiments.
AND TCR transgene fails to correct developmental defects in DN-IkTg thymocytes
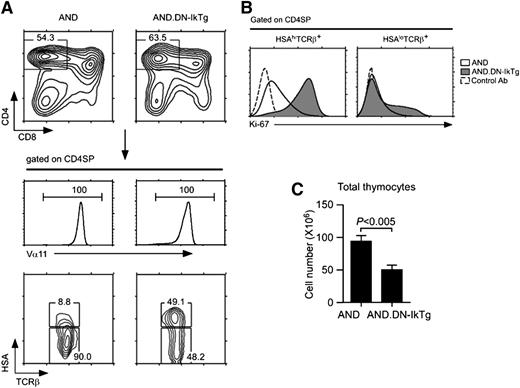
To test whether transgenic TCR can relieve the developmental arrest of DN-IkTg CD4SP cells, we introduced the MHCII-restricted AND TCR into DN-IkTg mice (AND.DN-IkTg). As expected, both AND and AND.DN-IkTg thymocytes exclusively generated CD4SP cells (Figure 6A, top), and all of them were positive for the clonotypic TCR Vα11 (Figure 6A, middle). Notably, AND.DN-IkTg CD4SP cells still contained a large population of HSAhiTCRβhi cells, which is characteristic of a DN-IkTg phenotype (Figure 6A, bottom). Furthermore, most of these cells were Ki-67 positive, suggesting that the AND TCR transgene failed to block aberrant proliferation of HSAhiTCRβhi cells (Figure 6B, left). The few mature CD4 cells that had escaped this developmental arrest, however, were Ki-67–negative even in the presence of Ik7 (Figure 6B, right). Therefore, DN-IkTg’s effect on cell proliferation was largely limited to a specific developmental stage. In addition, total thymocyte numbers were significantly reduced in AND.DN-IkTg mice, indicating that DN-IkTg impaired T-cell development even in TCR transgenic cells (Figure 6C). Thus, transgenic TCR expression is insufficient to replace Ikaros function during T-cell development.
AND TCR transgene fails to rescue developmental arrest of DN-IkTg CD4SP thymocytes. (A) Thymocyte profiles of AND and AND.DN-IkTg mice. Total thymocytes were assessed for CD4/CD8 expression, and TCR Vα11 expression was determined on gated CD4SP cells. HSA and TCRβ expression was assessed on Vα11hi CD4SP thymoycytes. Data are representative of at least 7 independent experiments with 7 AND and 9 AND.DN-IkTg mice. (B) Intracellular Ki-67 staining in HSAhi and HSAlo TCRβ+ CD4SP thymocytes of AND and AND.DN-IkTg mice. Data are representative of 3 independent experiments. (C) Total thymocyte numbers from AND and AND.DN-IkTg mice. The graph shows the mean ± SEM from 7 independent experiments with 7 AND and 9 AND.DN-IkTg mice.
AND TCR transgene fails to rescue developmental arrest of DN-IkTg CD4SP thymocytes. (A) Thymocyte profiles of AND and AND.DN-IkTg mice. Total thymocytes were assessed for CD4/CD8 expression, and TCR Vα11 expression was determined on gated CD4SP cells. HSA and TCRβ expression was assessed on Vα11hi CD4SP thymoycytes. Data are representative of at least 7 independent experiments with 7 AND and 9 AND.DN-IkTg mice. (B) Intracellular Ki-67 staining in HSAhi and HSAlo TCRβ+ CD4SP thymocytes of AND and AND.DN-IkTg mice. Data are representative of 3 independent experiments. (C) Total thymocyte numbers from AND and AND.DN-IkTg mice. The graph shows the mean ± SEM from 7 independent experiments with 7 AND and 9 AND.DN-IkTg mice.
DN-IkTg CD4SP thymocytes are MHCII-restricted postselection thymocytes
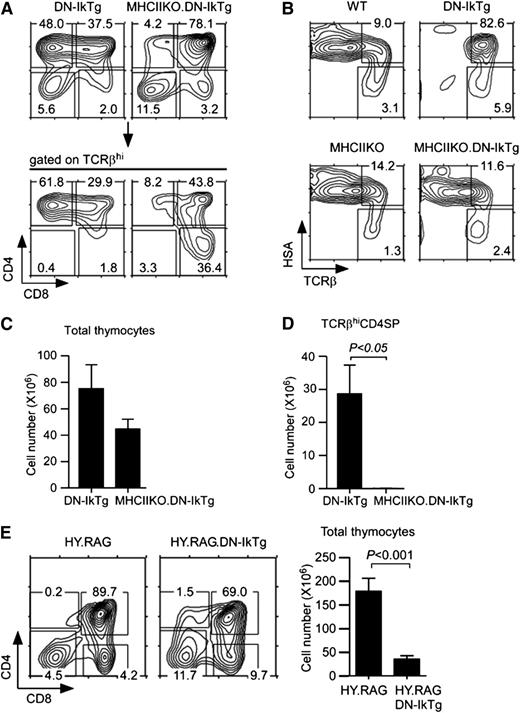
On the basis of these results, we hypothesized that Ikaros would promote differentiation but would suppress proliferation of positively-selected thymocytes. If this were the case, Ikaros should act downstream of positive-selecting TCR signals and DN-IkTg’s effect would be dependent on TCR engagement. To test this idea, we generated MHCII-deficient DN-IkTg mice (MHCIIKO.DN-IkTg) to disable positive selection of CD4 lineage cells. Strikingly, in such MHCIIKO.DN-IkTg mice, CD4SP cell accumulation was dramatically reduced (Figure 7A), and the developmental arrest of HSAhiTCRβhi transitional cells was lifted (Figure 7B). Reduced CD4SP cell percentages translated into reduced overall thymocyte numbers and into a dramatic loss of CD4SP cell numbers compared with DN-IkTg mice (Figure 7C-D). These results indicate that intermediate phenotype DN-IkTg CD4SP cells are postselection thymocytes and that their accumulation is dependent on TCR/MHCII engagement.
Clonal expansion of postselection CD4+CD8lo intermediate cells in DN-IkTg thymocytes. (A) Thymocyte profiles of DN-IkTg and MHCIIKO.DN-IkTg mice. Contour plots show CD4/CD8 profiles of total and TCRβhi-gated thymocytes. Data are representative of 5 independent experiments with 6 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (B) Thymocyte differentiation in MHCIIKO.DN-IkTg mice. HSA vs TCRβ profiles of WT, DN-IkTg, MHCIIKO, and MHCIIKO.DN-IkTg mice. Data are representative of 4 independent experiments with 4 WT, 6 DN-IkTg, 5 MHCIIKO, and 5 MHCIIKO.DN-IkTg mice. (C) Total thymocyte numbers of DN-IkTg and MHCIIKO.DN-IkTg mice. The bar graph shows the mean ± SEM from 3 independent experiments with 10 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (D) TCRβhi CD4SP thymocyte numbers of age-matched DN-IkTg and MHCIIKO.DN-IkTg mice. The bar graph shows the mean ± SEM from 3 independent experiments with 10 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (E) Thymocyte profiles and cell numbers of HY.RAG.DN-IkTg mice. CD4/CD8 profiles are representative of 5 independent experiments. The bar graph shows the mean ± SEM from 5 independent experiments with 6 HY.RAG female and 9 HY.RAG.DN-IkTg female mice.
Clonal expansion of postselection CD4+CD8lo intermediate cells in DN-IkTg thymocytes. (A) Thymocyte profiles of DN-IkTg and MHCIIKO.DN-IkTg mice. Contour plots show CD4/CD8 profiles of total and TCRβhi-gated thymocytes. Data are representative of 5 independent experiments with 6 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (B) Thymocyte differentiation in MHCIIKO.DN-IkTg mice. HSA vs TCRβ profiles of WT, DN-IkTg, MHCIIKO, and MHCIIKO.DN-IkTg mice. Data are representative of 4 independent experiments with 4 WT, 6 DN-IkTg, 5 MHCIIKO, and 5 MHCIIKO.DN-IkTg mice. (C) Total thymocyte numbers of DN-IkTg and MHCIIKO.DN-IkTg mice. The bar graph shows the mean ± SEM from 3 independent experiments with 10 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (D) TCRβhi CD4SP thymocyte numbers of age-matched DN-IkTg and MHCIIKO.DN-IkTg mice. The bar graph shows the mean ± SEM from 3 independent experiments with 10 DN-IkTg and 5 MHCIIKO.DN-IkTg mice. (E) Thymocyte profiles and cell numbers of HY.RAG.DN-IkTg mice. CD4/CD8 profiles are representative of 5 independent experiments. The bar graph shows the mean ± SEM from 5 independent experiments with 6 HY.RAG female and 9 HY.RAG.DN-IkTg female mice.
To further demonstrate a requirement for TCR/MHCII engagement in semimature CD4SP cell accumulation, we generated DN-IkTg mice expressing the MHCI-restricted HY TCR transgene. These mice were additionally rendered Rag2-deficient so that all developing thymocytes are selected by the HY TCR in an MHCI-dependent manner (HY.RAG.DN-IkTg). Notably, CD4SP cells were conspicuously absent in HY.RAG.DN-IkTg mice (Figure 7E). Positive selection of CD8SP cells, on the other hand, was not affected, even as total thymocyte numbers were significantly reduced (Figure 7E). Thus, aberrant accumulation of semimature CD4SP cells is dependent on MHCII-restricted TCR selection.
Collectively, here we identified a new developmental decision point at the Cd4+Cd8– intermediate stage that requires Ikaros function to guard positive-selecting TCR signals from inducing clonal expansion and proliferation, and to preserve a diverse and balanced postselection TCR repertoire (supplemental Figure 6).
Discussion
T-cell development in the thymus is dependent on a careful balance between differentiation and expansion of a useful TCR repertoire. Because both events are driven by TCR signaling, it is evident that there must be a mechanism to discriminate the need for differentiation vs proliferation. We now identify Ikaros as a critical component in this decision process and specifically important for intermediate thymocytes undergoing positive selection. In thymocytes expressing a DN Ikaros isoform, we found that TCR-signaled DP cells failed to differentiate into mature thymocytes. Instead, positive-selected cells proliferated and expanded into an oligoclonal CD4SP population, with a mixed phenotype of immature and mature differentiation markers. Importantly, excessive proliferation did not result in tumor because these cells concurrently underwent massive apoptosis. These results suggest that Ikaros is a critical regulator of T-cell development by controlling the downstream instructive effects of TCR signaling in immature DP thymocytes.
Thymocyte differentiation is a multistep process that is compartmentalized into distinct development stages.1,3 Progression through these different compartments is driven by a series of transcription factors, and Ikaros had been proposed to play a critical role in this process.11 Ikaros-null mice are defective in fetal T-cell development, have dramatically reduced thymocyte numbers at birth, and display an early developmental arrest in thymocyte differentiation.18 In addition, Ikaros deficiency results in a skewed CD4/CD8 ratio of thymocytes with increased CD4SP percentages.12 Mechanistically, it was proposed that Ikaros deficiency lowered the TCR signaling threshold and thus enhanced generation of CD4SP cells.12,18,19 Although Ikaros null T cells are indeed hyper-responsive to TCR signaling, how lowering the TCR signaling threshold would selectively enhance CD4SP generation remains to be explained. Nevertheless, experimental data favored a role for Ikaros in CD4/CD8 lineage choice because it appeared that Ikaros deficiency not only promoted CD4SP cell differentiation but could even induce CD4 lineage redirection of MHCI-restricted thymocytes. Along this line, MHCI-restricted F5 TCRTg immature thymocytes differentiated into CD4SP cells in Ikaros-null mice,41 and a similar population of lineage redirected CD4SP cells had been observed in Ikaros-null HY TCRTg thymocytes.12,20 Interestingly, such redirected CD4SP cells expressed clonotypic MHCI-restricted TCRs but were phenotypically and functionally immature, so that they were labeled as “CD4-like” thymocytes.20 Again, the molecular basis for the appearance of such redirected cells remained unsolved. Our study now provides an explanation for increased CD4SP cell percentages and skewed CD4/CD8 ratios in Ikaros-deficient mice. Using DN Ikaros expression, we identified the expanded population of CD4SP cells as developmentally transitional thymocytes that correspond to phenotypically CD4+CD8lo and transcriptionally Cd4+Cd8– intermediate cells in WT mice.9 Importantly, our finding provides explanation for the exclusive CD4SP feature of this expanded population, because intermediate cells terminate only Cd8 and not Cd4 transcription.1,36 It also explains why lineage-redirected Ikaros-null F5 or HY TCR transgenic CD4SP cells are never found in the periphery, because intermediate cells are not functionally competent for thymic export and migration.20,41
Using DN-IkTg mice to assess Ikaros function in T-cell development provided us with distinct advantages over using Ikaros-null mice. For example, accumulation of semimature CD4SP cells is more pronounced in DN-IkTg thymocytes than in Ikaros-null thymocytes, so that we consider DN-IkTg mice to be a more potent and faithful reporter of Ikaros requirement than Ikaros-deficient mice. DN-IkTg mice possibly display a more pronounced effect because DN-Ikaros would also suppress function of other Ikaros family members, such as Aiolos or Helios, in addition to Ikaros function itself. Along these lines, Aiolos has been reported to be highly expressed in immature thymocytes and to overlap with Ikaros function.30,38,42 Thus, we consider it likely that Ikaros-null thymocytes retain some Ikaros activity because of redundant activity of Aiolos and other Ikaros family members. This would be in contrast to DN-IkTg mice, where presumably the function of all Ikaros family members is suppressed. However, whether this is indeed the case remains to be tested.
We initially considered the possibility of DN-IkTg CD4SP cells being either immature DN cells with deregulated Cd4 repression43 or being immature SP cells expressing CD4 instead of CD8.41 However, their high levels of TCRβ expression and their conspicuous absence under MHCII-deficient conditions suggest they are postselection thymocytes. Because of their partially immature state, these cells are not long-lived. Moreover, the loss of semimature CD4SP cells in MHCII-deficient DN-IkTg mice may be further amplified by the lack of persistent TCR signaling that would otherwise maintain survival of aberrant CD4SP intermediate cells in vivo.3,9 In MHCII-deficient DN-IkTg CD4SP, neither of these pathways is operating. Consequently, DN-IkTg CD4SP thymocytes are postselection cells that are dependent on TCR engagement, but they fail to survive and mature because of massive cell death in the absence of Ikaros.
Finally, we were perplexed that germline-dominant Ikaros, but not T cell–specific DN-IkTg induces thymoma. In humans, Ikaros mutations are highly associated with T- and B-ALL23 and were identified as strong predictors of an increased likelihood of relapse in children with B-ALL.44 In addition, loss of Ikaros function is also associated with BCR-ABL1 lymphoblastic leukemia, as well as a common genetic lesion in Philadelphia chromosome–positive adult ALL.45 Notably, a large proportion of such Ikaros-associated tumors had a second activating mutation in Notch.46 Thus, deregulation of Notch expression could potentially provide the missing survival signals in DN-Ikaros–expressing cells. Along this line, an Ikaros single-point mutation that disrupts the N-terminal DNA binding domain (plastic mice) leads to T cell lymphoma in mice,47 and analysis of these tumors revealed that a majority (70%) had acquired a second activating mutation in Notch1.48 Also, mice expressing an Ikaros hypomorphic mutation (IkL/L) resulted in thymoma,49 and again, all tumors showed a strong signature of Notch activation. Collectively, combined deregulation of Ikaros and Notch could be the in vivo requirements for tumorigenesis. Therefore, the absence of tumor formation in proliferating CD4SP intermediate cells could be the lack of such activated Notch signaling in T cell–specific DN-IkTg mice.
In conclusion, we identify a new developmental checkpoint during thymocyte development that is created upon positive selection and that is controlled by Ikaros. We show that Ikaros function is required for positive selection, and that DP thymocytes are uniquely refractory to TCR-induced proliferation because of Ikaros function. Moreover, suppression of Ikaros function in signaled DP thymocytes (ie, intermediate cells) was critical to generate a diverse TCR repertoire and to promote thymocyte survival and differentiation. Mechanistically, we identified a series of molecules in the apoptosis pathway, such as Bcl2 and Bag3, as transcriptional targets for Ikaros whose functions need further clarification. Identification of other target genes that are downstream of Ikaros and induce uncontrolled proliferation is a target of further studies to understand Ikaros’s role in leukemia and lymphoma.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs A. Singer and X. Tai for insightful review of this manuscript, and D. L. Ligons for cell sorting and help with immunoblot analysis. The Ik7 cDNA was kindly provided by Dr S. Winandy (Boston University).
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Authorship
Contribution: K.W.T. and C.H. performed experiments, analyzed data, and contributed to writing the manuscript; M.A.L., J.-Y.P., G.Y.K., H.W.Y., H.R.K., A.J.S., and L.F. performed experiments and analyzed data; and J.-H.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.W.T. is the Laboratory of Molecular Immunogenetics, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD. The current affiliation for G.Y.K. is MassGeneral Hospital for Children, Boston, MA.
Correspondence: Jung-Hyun Park, Experimental Immunology Branch, National Cancer Institute, National Institutes of Health, Bldg 10, Room 5B17, 10 Center Dr, Bethesda, MD 20892; e-mail: parkhy@mail.nih.gov.
References
Author notes
K.W.T. and C.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal