Key Points
The endothelial leukocyte receptor VCAM-1 triggers opening of endothelial junctions via dissociation of VE-PTP from VE-cadherin.
VCAM-1 and VEGF signaling use a similar signaling pathway to trigger the dissociation of VE-PTP from VE-cadherin.
Abstract
The vascular endothelial (VE) receptor protein tyrosine phosphatase (VE-PTP) associates with VE-cadherin and supports endothelial cell contact integrity. This complex is rapidly dissociated by adhesion of leukocytes to endothelial cells or by vascular endothelial growth factor. We have shown recently that this dissociation is indeed required for the opening of endothelial cell contacts during leukocyte extravasation in vivo. The leukocyte receptor and signaling mechanism that stimulates VE-cadherin/VE-PTP dissociation are unknown. Here, we identify vascular cell adhesion molecule 1 as the relevant receptor for lymphocytes in this process. As signaling steps downstream of this receptor, we determined the activation of Rac1, the generation of reactive oxygen species by nicotinamide adenine dinucleotide phosphate oxidase and the activation of the redox-sensitive tyrosine kinase Pyk2 as essential for VE-cadherin/VE-PTP dissociation. These signaling steps are also required for the dissociation induced by VE growth factor. Searching for the molecular mechanism of complex dissociation, we found that a model substrate of VE-PTP represented by a tyrosine-phosphorylated peptide of Tie-2 dissociates VE-PTP from VE-cadherin when introduced with the help of a Tat peptide. We suggest that lymphocyte binding to vascular cell adhesion molecule 1 triggers a signaling process that enables a VE-PTP substrate to dissociate VE-PTP from VE-cadherin, thereby facilitating efficient transmigration.
Introduction
During inflammation, leukocytes extravasating from the blood into tissues have to migrate through the blood vessel wall. This is achieved by first binding to various cytokine-induced adhesion receptors on endothelial cells followed by inducing openings in the endothelial barrier.1 Although these openings can be transcellular, the vast majority of leukocytes use a paracellular route through endothelial junctions, as was documented in vitro2 and more recently in vivo.3,4 Vascular endothelial (VE)-cadherin is a junctional adhesion molecule that is of vital importance for the integrity of endothelial cell junctions.5,6 Blocking of VE-cadherin adhesion by antibodies is sufficient to destabilize endothelial junctions in vivo and to increase leukocyte extravasation.7,8 Furthermore, replacing the VE-cadherin-β-catenin-α-catenin complex by a VE-cadherin-α-catenin fusion protein in knock-in mice stabilizes endothelial junctions efficiently and strongly inhibits leukocyte extravasation in various tissues.4 This highlights the importance of regulating VE-cadherin function during leukocyte extravasation. It has been shown that tyrosine phosphorylation of VE-cadherin correlates with destabilization of endothelial junctions9-11 and participates in vitro in the transmigration of leukocytes through endothelial cell monolayers.12,13
VE protein tyrosine phosphatase (VE-PTP) is an endothelial-specific receptor PTP14-16 that associates with VE-cadherin. This association is mediated by extracellular protein domains and enhances VE-cadherin–mediated adhesion in mammalian cells in a phosphatase-activity–dependent manner.17 Apart from VE-cadherin, VE-PTP also interacts with and regulates the angiopoietin receptor Tie-2.14,18 Also, the VE growth factor receptor-2 (VEGFR-2) has been reported to interact with VE-PTP.19 The phosphatase activity of VE-PTP is critically important for blood vessel development, as intracellular truncation or deletion of VE-PTP causes embryonic lethality shortly before 10 days of gestation,15,16 and detachment of VE-PTP from Tie-2 leads to severe remodeling defects of the blood vasculature.18
In cultured endothelial cells, VE-PTP is necessary for the proper maintenance of endothelial cell contact integrity.20 Silencing of VE-PTP leads to increased cell permeability and increased leukocyte migration through endothelial cell monolayers. The first indirect evidence for a role of VE-PTP in leukocyte diapedesis was based on the finding that adhesion of neutrophils and lymphocytes to endothelial cells each triggered the rapid dissociation of VE-PTP from VE-cadherin.20 Because VEGF, a potent permeability-inducing factor, could also dissociate VE-PTP from VE-cadherin, we suggested that this dissociation of an adhesion supporting phosphatase would be a prerequisite for the opening of endothelial junctions. We could recently show that this dissociation also occurs in vivo in the mouse by lipopolysaccharide-induced leukocyte extravasation and by VEGF.21 Furthermore, we could demonstrate that preventing the dissociation of VE-PTP from VE-cadherin strongly inhibited leukocyte extravasation in vivo as well as the induction of vascular permeability.21 This mechanism was demonstrated with knock-in mice expressing modified fusion proteins of VE-cadherin and VE-PTP containing additional protein domains that allowed stabilization of their association by a chemical heterodimerizer. In these mice, intravenous application of the heterodimerizer strongly inhibited neutrophil extravasation in the inflamed cremaster and the lipopolysaccharide-inflamed lung. This documented that the dissociation of VE-PTP from VE-cadherin is indeed necessary in vivo for the opening of endothelial cell contacts during leukocyte extravasation.21 Despite the essential role of this mechanism for leukocyte extravasation, the endothelial leukocyte receptor and the signaling mechanism that trigger VE-PTP/VE-cadherin dissociation are still unknown.
Here, we show that lymphocytes trigger the dissociation of VE-PTP from VE-cadherin via binding to the VE adhesion receptor-1 (VCAM-1). The signaling pathway involved in this process requires the GTPase Rac1, reactive oxygen species (ROS), and the kinase Pyk2. Investigating the actual dissociation process in more detail revealed that a model substrate of VE-PTP is able to detach the phosphatase from VE-cadherin.
Material and methods
Reagents and antibodies
The following reagents were used: Dynabeads and dichlorofluorescein (DCF) (Invitrogen); NSC23766, diphenyleneiodonium chloride (DPI), pyrazolopyrimidine 2 (PP2), and SU6566 (Calbiochem); U0126 and U0124 (Cell Signaling Technology); PF431396 and PF573228 (Tocris); N-acetylcysteine (NAC) (Sigma-Aldrich); VAS2870 (Enzo); VEGF165 (R&D Systems); tumor necrosis factor-alpha (TNF-α) (Peprotech); and the cell-penetrating Tat-Tie2 peptides (either phosphorylated or not at Y992) covered amino acids 984-996 of human Tie-2 (identical with the mouse sequence from aa 982-994), extended for the Tat peptide at the N terminus (GRKKRRQRRRPQGLSRGQEVYVKKT) (Thermo Fisher Scientific and Selleck Chemicals). Vectors encoding Tat-Rac1 fusion proteins22 were a gift from C. Laudanna. Antibodies against the following antigens were used: rat mAb 11D4.1 against murine VE-cadherin,8 rabbit and goat pAb VE-PTP-C against VE-PTP,17 rat mAb 109.1 against murine VE-PTP,15 rat mAb 6C7.1 against murine VCAM-1,23 rat mAb YNI.1 against murine ICAM-1,24 Pyk2 and phospho-Pyk2 pAbs (Cell Signaling Technology), anti-phosphotyrosine mAb 4G10 (Millipore), and Rac1 mAb (BD Transduction Labs). Peroxidase-labeled secondary antibodies were obtained from Dianova.
Cell lines and primary cells
Murine bEnd.5 endothelioma cells and endothelioma cells established from VE-PTPmut/mut and VE-PTP+/+ mouse embryos15 were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM of l-glutamine, 1 mM of sodium pyruvate, 1% nonessential amino acids, and 1% penicillin/streptomycin (PAA Laboratories). Primary lung microvascular endothelial cells were isolated from wild-type mice as described previously25 and were cultivated in endothelial cell growth medium containing 20% fetal calf serum, 50 µg/mL of heparin, and 20 µg/mL of endothelial cell growth supplement. OTII-Ova T cells expressing α and β chains of a T-cell receptor specific for peptide 323-339 of ovalbumin were isolated from OTII mice (The Jackson Laboratory) and propagated as described.26 T cells were activated by co-culturing with irradiated B6 splenocytes plus 1 µg/mL of OVA323-339 peptide for 3 days followed by culturing 2 to 4 days in the presence of IL-2 before use. Chinese hamster ovary (CHO) cells were triple-transfected with VE-cadherin, VEGFR-2, and inducible full-length wild-type VE-PTP or the respective C/S substrate-trapping mutant of VE-PTP in a similar way as described,17 except that VE-PTP was full length and the mutant was a C/S instead of an R/A mutant. Expression of VE-PTP was induced with mifepristone for 10 hours at 37°C.
Cell treatments
Highly confluent bEnd.5 cells or primary endothelial cells (14 days in culture) were stimulated for 18 hours with 5 nM of TNF-α. Cells were incubated for 15 minutes with 3 × 107 antigen-stimulated T cells. Adhering cells were removed by washing 2 times with phosphate-buffered saline. Pharmacological inhibitors were added for 30 minutes and were washed away before leukocyte adhesion (25 µM of NSC23766, 2 µM of DPI, 10 µM of PP2, 5 µM of SU6566, 10 µM of U0126 or U0124, 5 µM of PF431396 or PF573228, 1 mM of NAC and 10 µM of VAS2870). The Tat-Tie-2 peptides were incubated with bEnd.5 cells for 5 minutes at a concentration of 10 µM before immunoprecipitation analysis. The Tat-Rac1-CA and Tat-Rac1-wild-type polypeptides were incubated for 20 minutes at a concentration of 2 µM. For antibody-mediated crosslinking, antibodies were bound to sheep-anti rat IgG-coupled dynabeads (30 µg Ab per 100 µL of beads) overnight and washed according to the manufacturer’s protocol (Invitrogen). Antibody-loaded beads (30 µL/mL) were incubated with confluent bEnd.5 cells for 15 minutes. For antibody blockade, bEnd.5 cells were preincubated with 100 µg/mL of antibody for 15 minutes at 37°C. VEGF stimulation was performed with 100 ng/mL of recombinant VEGF for 30 minutes. ROS formation was monitored with a fluorescent DCF sensor according to the manufacturer's procedure (Invitrogen).
Immunoprecipitation and immunoblotting
For coimmunoprecipitations, cells were lysed in lysis buffer (20 mM of imidazole pH 6.8, 100 mM of NaCl, 2 mM of CaCl2, and 1% Triton X-100) with complete EDTA-free protease inhibitor cocktail (Roche) for 30 minutes at 4°C. Lysates were cleared by centrifugation for 30 minutes at 4°C and incubated for 3 hours at 4°C with protein A/G–sepharose loaded with the respective antibodies. Sepharose beads were washed 5 times with lysis buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Cell lysates and immunoprecipitations were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose (Schleicher & Schuell) by wet blotting.
Measurement of Rac1 activity
Active (guanosine triphosphate [GTP]–bound) Rac1 was precipitated from serum-starved bEnd.5 lysates with an immobilized glutathione-S-transferase (GST) fusion protein of the purified Rac1-binding domain of the p21-activated kinase PAK (PAK[PBD]).27 The GST-PAK fusion protein was expressed and isolated from Escherichia coli and handled according to the manufacturer's procedures (GE Healthcare).
Results
T cells trigger the dissociation of VE-PTP from VE-cadherin via VCAM-1
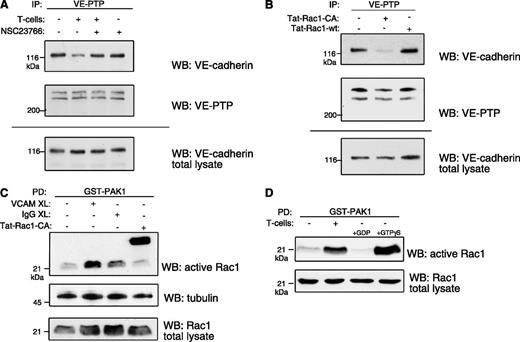
Searching for the endothelial receptor that triggers neutrophil- and lymphocyte-induced dissociation of VE-PTP from VE-cadherin, we have excluded before E- and P-selectin, ICAM-1, ICAM-2, PECAM-1 and CD99.20 Initially, VCAM-1 had not been analyzed, because it is not a major player in neutrophil extravasation in all tissues across species. However, because lymphocytes require VCAM-1 for transendothelial migration, we tested whether crosslinking of VCAM-1 would trigger VE-PTP/VE-cadherin dissociation. Beads loaded with the monoclonal antibody (mAb) 6C7 against VCAM-1 were incubated with bEnd.5 mouse endothelioma cells for 5 minutes and then for 15 minutes. As shown in Figure 1A, this treatment dissociated VE-PTP from VE-cadherin as tested in coimmunoprecipitations of VE-cadherin with VE-PTP. The dissociation effect was similar as seen with T cells binding to bEnd.5 (Figure 1A). In this experiment and in all subsequent experiments, the same immunoblot filter that had been analyzed for coprecipitated VE-cadherin was analyzed for VE-PTP to control for similar amounts of precipitated VE-PTP (Figure 1A). Furthermore, we ruled out potential degradation of VE-cadherin by immunoblotting cell lysate aliquots (Figure 1A, bottom panel). VCAM-1–induced dissociation of VE-PTP from VE-cadherin could also be shown in primary murine lung microvascular endothelial cells (Figure 1B).
VCAM-1 ligation dissociates VE-PTP from VE-cadherin. (A) VCAM-1 crosslinking (XL) induces VE-cadherin/VE-PTP dissociation. Either antigen-stimulated T cells or anti-VCAM-1–coupled dynabeads (mAb 6C7) were added to confluent TNF-α–stimulated bEnd.5 endothelioma cells for 5 or 15 minutes. VE-PTP was immunoprecipitated from lysates, and either coprecipitated VE-cadherin (WB: VE-cadherin) or precipitated VE-PTP (WB: VE-PTP) was detected by immunoblotting. To exclude proteolysis as a potential reason for the weakening of the VE-cadherin signal, aliquots of cell lysates were set aside and kept under identical conditions as lysates subjected to immunoprecipitations before direct analysis by immunoblotting (WB: VE-cadherin total lysate). (B) VCAM-1 crosslinking dissociates VE-cadherin and VE-PTP in murine primary endothelial cells. Confluent TNF-α–stimulated cells were incubated with anti-VCAM-1– or control IgG–coupled dynabeads for 15 minutes. Coprecipitated VE-cadherin was detected by immunoblotting as described above. (C) Anti–VCAM-1 blockade inhibits T-cell–induced dissociation. TNF-α–stimulated bEnd.5 cells were either incubated with control IgG– or anti-VCAM-1 antibody–loaded beads (first 2 lanes), or bEnd.5 cells were preincubated with anti-VCAM-1 antibody or no antibody. This was followed by incubation with T cells (last 2 lanes) and subsequent coimmunoprecipitation analysis. (D) ICAM-1 is not required for VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were either incubated with beads loaded with control IgG, anti–VCAM-1 mAb, or anti-ICAM-1 mAb (first 3 lanes), or the endothelial cells were preincubated with anti-VCAM-1 mAb or with anti-ICAM-1 mAb, followed by incubation with T cells (lanes 4-5) and subsequent coimmunoprecipitation analysis.
VCAM-1 ligation dissociates VE-PTP from VE-cadherin. (A) VCAM-1 crosslinking (XL) induces VE-cadherin/VE-PTP dissociation. Either antigen-stimulated T cells or anti-VCAM-1–coupled dynabeads (mAb 6C7) were added to confluent TNF-α–stimulated bEnd.5 endothelioma cells for 5 or 15 minutes. VE-PTP was immunoprecipitated from lysates, and either coprecipitated VE-cadherin (WB: VE-cadherin) or precipitated VE-PTP (WB: VE-PTP) was detected by immunoblotting. To exclude proteolysis as a potential reason for the weakening of the VE-cadherin signal, aliquots of cell lysates were set aside and kept under identical conditions as lysates subjected to immunoprecipitations before direct analysis by immunoblotting (WB: VE-cadherin total lysate). (B) VCAM-1 crosslinking dissociates VE-cadherin and VE-PTP in murine primary endothelial cells. Confluent TNF-α–stimulated cells were incubated with anti-VCAM-1– or control IgG–coupled dynabeads for 15 minutes. Coprecipitated VE-cadherin was detected by immunoblotting as described above. (C) Anti–VCAM-1 blockade inhibits T-cell–induced dissociation. TNF-α–stimulated bEnd.5 cells were either incubated with control IgG– or anti-VCAM-1 antibody–loaded beads (first 2 lanes), or bEnd.5 cells were preincubated with anti-VCAM-1 antibody or no antibody. This was followed by incubation with T cells (last 2 lanes) and subsequent coimmunoprecipitation analysis. (D) ICAM-1 is not required for VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were either incubated with beads loaded with control IgG, anti–VCAM-1 mAb, or anti-ICAM-1 mAb (first 3 lanes), or the endothelial cells were preincubated with anti-VCAM-1 mAb or with anti-ICAM-1 mAb, followed by incubation with T cells (lanes 4-5) and subsequent coimmunoprecipitation analysis.
To test whether VCAM-1 is necessary for T-cell–induced VE-PTP/VE-cadherin dissociation, we blocked T-cell binding with the mAb 6C7. As shown in Figure 1C, this mechanism abrogated T-cell–induced VE-PTP/VE-cadherin dissociation. As a negative control, crosslinking of ICAM-1 with a mAb did not trigger VE-PTP/VE-cadherin dissociation, and blocking of ICAM-1 had no inhibitory effect on T-cell–induced detachment of both proteins (Figure 1D). Thus, VCAM-1 is an essential receptor for this T-cell–induced dissociation mechanism.
Rac1 activation is required for VE-cadherin/VE-PTP dissociation
Because clustering of VCAM-1 activates the small GTPase Rac1,1,28,29 we used the selective pharmacological Rac1 inhibitor NSC23766230 and found that it prevents T-cell–induced VE-cadherin/VE-PTP dissociation when preincubated with bEnd.5 cells (Figure 2A). In addition, we found that a cell-penetrating form of a constitutively active mutant of Rac1 (Tat-Rac1-L61)22 was sufficient to dissociate VE-PTP from VE-cadherin in bend.5 cells (Figure 2B). To confirm that VCAM-1 crosslinking as well as T-cell adhesion indeed activated Rac1 in our experimental setting, we examined Rac1 activation in bEnd.5 cells by GST-PAK affinity precipitations (Figure 2C-D). Our results demonstrate that activation of Rac1 is necessary and sufficient to trigger the dissociation of VE-PTP from VE-cadherin.
Rac1 activity is required for dissociation of VE-cadherin and VE-PTP. (A) Rac1 inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were treated with the Rac1 inhibitor NSC23766 (25 μM) before incubation with antigen-stimulated T cells, followed by coimmunoprecipitation of VE-cadherin via VE-PTP (analogous to Figure 1). (B) Rac1 activation dissociates VE-PTP from VE-cadherin. bEnd.5 cells were incubated with a constitutively active mutant Rac1 fusion protein (Tat-Rac1-CA) or a wild-type fusion protein (Tat-Rac1-wt), followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (C) VCAM-1 crosslinking (XL) activates Rac1. Anti-VCAM-1– or control IgG–coupled dynabeads or a Tat-Rac1-CA fusion protein (as a positive control) were incubated with TNF-α–stimulated bEnd.5 cells, followed by pulldown of active (GTP-bound) Rac1 with the purified Rac1-binding domain of PAK1 (as GST fusion protein). (D) TNF-α–stimulated bEnd.5 cells were incubated with or without T cells, and subsequently active Rac1 was analyzed as in panel C. For controls, GDP or nonhydrolysable GTPγS was added (right 2 lanes).
Rac1 activity is required for dissociation of VE-cadherin and VE-PTP. (A) Rac1 inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were treated with the Rac1 inhibitor NSC23766 (25 μM) before incubation with antigen-stimulated T cells, followed by coimmunoprecipitation of VE-cadherin via VE-PTP (analogous to Figure 1). (B) Rac1 activation dissociates VE-PTP from VE-cadherin. bEnd.5 cells were incubated with a constitutively active mutant Rac1 fusion protein (Tat-Rac1-CA) or a wild-type fusion protein (Tat-Rac1-wt), followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (C) VCAM-1 crosslinking (XL) activates Rac1. Anti-VCAM-1– or control IgG–coupled dynabeads or a Tat-Rac1-CA fusion protein (as a positive control) were incubated with TNF-α–stimulated bEnd.5 cells, followed by pulldown of active (GTP-bound) Rac1 with the purified Rac1-binding domain of PAK1 (as GST fusion protein). (D) TNF-α–stimulated bEnd.5 cells were incubated with or without T cells, and subsequently active Rac1 was analyzed as in panel C. For controls, GDP or nonhydrolysable GTPγS was added (right 2 lanes).
T-cell–induced ROS production is required for VE-cadherin/VE-PTP dissociation
Because VCAM-1–triggered activation of Rac1 supports the production of ROS,28,31,32 we tested whether scavenging of ROS with the antioxidant NAC would interfere with T-cell–induced dissociation of VE-cadherin from VE-PTP. This was indeed the case (Figure 3A). We confirmed that T cells induced the production of endothelial ROS in our experimental setting by using an intracellular fluorescent ROS sensor (Figure 3B). Characterizing the source of ROS production, we found that preincubation of bEnd.5 cells with the flavoenzyme inhibitor DPI prevented the T-cell–triggered dissociation of VE-cadherin and VE-PTP (Figure 3C). In addition, DPI also inhibited the detachment of VE-PTP from VE-cadherin triggered by VCAM-1 crosslinking (Figure 3D). To further clarify the enzymatic source of ROS production, we used the specific reduced nicotinamide adenine dinucleotide phosphate oxidase (NOX) inhibitor VAS2870. Also, this inhibitor blocked the T-cell–induced detachment of VE-PTP from VE-cadherin in bEnd.5 cells (Figure 3E), and VCAM-1 induced detachment of both proteins in primary mouse lung microvascular endothelial cells (Figure 3F). We conclude that ROS produced by NOXs are essential mediators in leukocyte-triggered cell contact regulation.
Endothelial ROS production induces VE-cadherin/VE-PTP dissociation. (A) Scavenging of ROS inhibits T-cell–induced VE-cadherin/VE-PTP dissociation. Preincubation of TNF-α–stimulated bEnd.5 cells with or without the ROS scavenger NAC (1 mM) (as indicated above) was followed by incubation with T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) T-cell adhesion induces ROS production in endothelial cells. TNF-α–stimulated bEnd.5 cells were loaded with the fluorescent ROS indicator DCF and were subsequently incubated either with H2O2 (as a positive control) or with T cells, followed by recording intracellular fluorescence. (C) Inhibition of ROS production prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were pretreated with 2 μM of the flavoprotein inhibitor DPI, followed by incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) The same process was done as in panel C, but bEnd.5 cells were stimulated with anti-VCAM-1–loaded beads (VCAM XL) instead of T cells. (E) NOX inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the specific NOX inhibitor VAS2870 (10 μM) and were subsequently analyzed as was done in panel C. (F) NOX inhibition prevents VE-cadherin/VE-PTP dissociation in murine primary lung endothelial cells. TNF-α–stimulated cells were pretreated with 10 μM of the VAS2870 NOX inhibitor, followed by stimulation with anti-VCAM-1–loaded beads (VCAM XL) and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis.
Endothelial ROS production induces VE-cadherin/VE-PTP dissociation. (A) Scavenging of ROS inhibits T-cell–induced VE-cadherin/VE-PTP dissociation. Preincubation of TNF-α–stimulated bEnd.5 cells with or without the ROS scavenger NAC (1 mM) (as indicated above) was followed by incubation with T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) T-cell adhesion induces ROS production in endothelial cells. TNF-α–stimulated bEnd.5 cells were loaded with the fluorescent ROS indicator DCF and were subsequently incubated either with H2O2 (as a positive control) or with T cells, followed by recording intracellular fluorescence. (C) Inhibition of ROS production prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were pretreated with 2 μM of the flavoprotein inhibitor DPI, followed by incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) The same process was done as in panel C, but bEnd.5 cells were stimulated with anti-VCAM-1–loaded beads (VCAM XL) instead of T cells. (E) NOX inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the specific NOX inhibitor VAS2870 (10 μM) and were subsequently analyzed as was done in panel C. (F) NOX inhibition prevents VE-cadherin/VE-PTP dissociation in murine primary lung endothelial cells. TNF-α–stimulated cells were pretreated with 10 μM of the VAS2870 NOX inhibitor, followed by stimulation with anti-VCAM-1–loaded beads (VCAM XL) and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis.
Pyk2 activity is required for VE-cadherin/VE-PTP dissociation
Endothelial-derived ROS are known to activate tyrosine kinases,33 and various kinases are involved in the regulation of junctions. It has been shown that the mitogen-activated extracellular signal–regulated kinase (ERK) is phosphorylated after VCAM-1 ligation in endothelial cells,34 and this has been suggested to be relevant for the regulation of leukocyte transmigration.35 In addition, binding of leukocytes to endothelial cells activates Src.13 However, we found that neither the ERK inhibitor U0126 nor the Src inhibitors PP2 and SU6566 interfered with T-cell–induced VE-PTP/VE-cadherin dissociation (Figure 4A-B).
Kinase regulation of VE-cadherin and VE-PTP association. (A) ERK1/2 activation is not required for VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the ERK1/2 inhibitor U0126 (or the negative control substance U0124) (both at 10 μM), followed by incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) Src inhibition does not prevent VE-cadherin/VE-PTP dissociation. The same process was done as in panel A, but cells were treated with the Src inhbitors PP2 (10 μM) or SU6566 (5 μM).
Kinase regulation of VE-cadherin and VE-PTP association. (A) ERK1/2 activation is not required for VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the ERK1/2 inhibitor U0126 (or the negative control substance U0124) (both at 10 μM), followed by incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) Src inhibition does not prevent VE-cadherin/VE-PTP dissociation. The same process was done as in panel A, but cells were treated with the Src inhbitors PP2 (10 μM) or SU6566 (5 μM).
Pyk2 is an another tyrosine kinase capable of affecting endothelial cell contact stability.13,36 Therefore, we used the Pyk2 inhibitor PF431396 and found that preincubation of bend.5 cells with this compound abrogated T-cell–induced detachment of VE-PTP from VE-cadherin (Figure 5A). Because PF431396 also inhibits the Pyk2-related focal adhesion kinase (FAK), we examined if a FAK-specific inhibitor (PF573228) that does not affect Pyk2 would have a similar effect. As shown in Figure 5B, this FAK-specific inhibitor was unable to impair T-cell–stimulated VE-PTP/VE-cadherin detachment. Thus, whereas the VCAM-1–triggered kinases Erk1/2 and Src were irrelevant for the dissociation of VE-PTP from VE-cadherin, the kinase Pyk2 is necessary for this process. An additional, redundant role of FAK can presently not be excluded.
Pyk2 activity regulates VE-cadherin/VE-PTP dissociation. (A) Pyk2 inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the indicated concentrations of the Pyk2/FAK inhibitor PF431396, followed by the incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) FAK activity is not required for VE-cadherin/VE-PTP dissociation. Analysis done is analogous to panel A, comparing the inhibitory activity of the dual-specificity Pyk2/FAK inhibitor PF431396 (5 μM) and the monospecific FAK inhibitor PF573228 (5 μM). (C) Pyk2 acts downstream of NOX activation. TNF-α–stimulated bEnd.5 cells were preincubated with the NOX inhibitor VAS2870 (10 μM) and were subsequently stimulated with or without anti–VCAM-1 mAb-loaded beads (VCAM XL), followed by immunoprecipitation of Pyk2 and immunoblotting with a general antiphosphotyrosine mAb (WB: pTyr), antibodies against phosphorylated tyrosine 402 of Pyk2 (WB: pPyk2 [Tyr402]), or antibodies against Pyk2 (WB: Pyk2). Equivalent aliquots of total cell lysates were analyzed by immunoblotting for Pyk2 (WB: Pyk2 total lysate).
Pyk2 activity regulates VE-cadherin/VE-PTP dissociation. (A) Pyk2 inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the indicated concentrations of the Pyk2/FAK inhibitor PF431396, followed by the incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) FAK activity is not required for VE-cadherin/VE-PTP dissociation. Analysis done is analogous to panel A, comparing the inhibitory activity of the dual-specificity Pyk2/FAK inhibitor PF431396 (5 μM) and the monospecific FAK inhibitor PF573228 (5 μM). (C) Pyk2 acts downstream of NOX activation. TNF-α–stimulated bEnd.5 cells were preincubated with the NOX inhibitor VAS2870 (10 μM) and were subsequently stimulated with or without anti–VCAM-1 mAb-loaded beads (VCAM XL), followed by immunoprecipitation of Pyk2 and immunoblotting with a general antiphosphotyrosine mAb (WB: pTyr), antibodies against phosphorylated tyrosine 402 of Pyk2 (WB: pPyk2 [Tyr402]), or antibodies against Pyk2 (WB: Pyk2). Equivalent aliquots of total cell lysates were analyzed by immunoblotting for Pyk2 (WB: Pyk2 total lysate).
Investigating the order of signaling steps, we found that antibody mediated crosslinking of VCAM-1 on the surface of bEnd.5 cells could no longer induce Pyk2 phosphorylation in the presence of the NOX inhibitor VAS2870 (Figure 5C). Thus, Pyk2 acts downstream of Rac1 and NOX.
VEGFR-triggered VE-cadherin/VE-PTP dissociation involves Rac1 activation, endothelial ROS production, and Pyk2 activity
Because stimulation of endothelial cells with VEGF also triggers the dissociation of VE-PTP from VE-cadherin, we wanted to know if similar signaling mechanisms are involved as during lymphocyte adhesion. Indeed, we found that the Rac1 inhibitor NSC237662 (Figure 6A), the antioxidant NAC and the NOX inhibitor VAS2870 (Figure 6B), and the dual Pyk2/FAK inhibitor (Figure 6C) all interfered with VEGF-induced dissociation of VE-PTP from VE-cadherin. Again, ROS production was required for Pyk2 activation (Figure 6D). Collectively, these results show that the VEGFR and VCAM-1 trigger an analogous signaling pathway to dissociate VE-PTP from VE-cadherin.
VEGFR signaling via Rac1/ROS/Pyk2 triggers VE-cadherin/VE-PTP dissociation. (A) Rac1 inhibition prevents VEGF-induced dissociation. bEnd.5 cells were treated with the Rac1 inhibitor NSC23766 (25 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) NOX inhibition prevents VEGF-induced dissociation. bEnd.5 cells were treated with the antioxidant NAC (1 mM) or the NOX inhibitor VAS2870 (10 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (C) Pyk2 activity is required for VEGF-induced dissociation. bEnd.5 cells were pretreated with the dual Pyk2/FAK inhibitor PF431396 (5 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) VEGF-triggered activation of Pyk2 requires NOX activity. bEnd.5 cells were pretreated with the ROS scavenger NAC (1 mM) or the NOX inhibitor VAS2870 (10 μM) before incubation with VEGF, followed by immunoprecipitating Pyk2 and immunoblotting for phospho-Pyk2 (WB: phospho-Pyk2 total lysate). Equivalent aliquots of total cell lysates were analyzed by immunoblotting for Pyk2 (WB: Pyk2 total lysate).
VEGFR signaling via Rac1/ROS/Pyk2 triggers VE-cadherin/VE-PTP dissociation. (A) Rac1 inhibition prevents VEGF-induced dissociation. bEnd.5 cells were treated with the Rac1 inhibitor NSC23766 (25 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) NOX inhibition prevents VEGF-induced dissociation. bEnd.5 cells were treated with the antioxidant NAC (1 mM) or the NOX inhibitor VAS2870 (10 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (C) Pyk2 activity is required for VEGF-induced dissociation. bEnd.5 cells were pretreated with the dual Pyk2/FAK inhibitor PF431396 (5 μM) before incubation with VEGF, followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) VEGF-triggered activation of Pyk2 requires NOX activity. bEnd.5 cells were pretreated with the ROS scavenger NAC (1 mM) or the NOX inhibitor VAS2870 (10 μM) before incubation with VEGF, followed by immunoprecipitating Pyk2 and immunoblotting for phospho-Pyk2 (WB: phospho-Pyk2 total lysate). Equivalent aliquots of total cell lysates were analyzed by immunoblotting for Pyk2 (WB: Pyk2 total lysate).
A substrate of VE-PTP is able to detach the phosphatase in endothelial cells from VE-cadherin
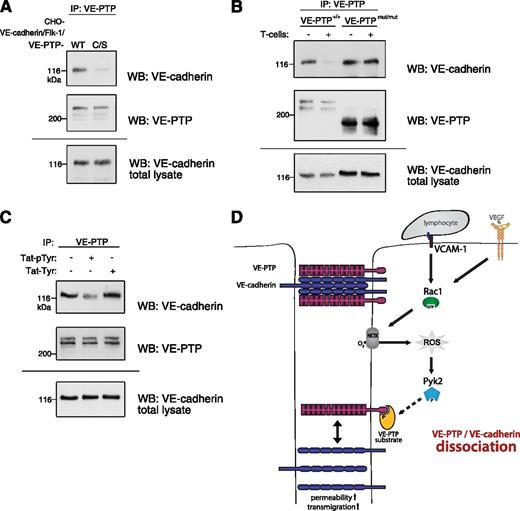
The next question we asked was how can the intracellular signaling process detach VE-PTP from VE-cadherin, although both proteins are interacting via extracellular domains.17 Surprisingly, we found that a C/S point-mutant of VE-PTP, with a cysteine-to-serine mutation in the active center of the phosphatase domain of VE-PTP, could no longer associate with VE-cadherin in transfected CHO cells, whereas nonmutated VE-PTP could easily attach to VE-cadherin (Figure 7A). Thinking of a reason for this unexpected result, we realized that the C/S mutation does not only destroy the dephosphorylation activity of the enzyme, but it also turns the phosphatase into a trapping mutant that can still bind substrates but cannot release them anymore because of the defective dephosphorylation activity. Thus, a VE-PTP substrate might stably bind to the newly synthesized VE-PTP C/S mutant even before it associates with VE-cadherin and thereby interfere with the attachment of VE-PTP to VE-cadherin.
Binding of a phosphorylated VE-PTP substrate induces VE-cadherin/VE-PTP dissociation. (A) VE-cadherin/VE-PTP association is severely reduced in CHO cells expressing a VE-PTP substrate-trapping (C/S) mutant compared with wild-type (WT) VE-PTP. CHO cells triple-transfected with VE-cadherin, VEGFR-2, and either the WT form or the C/S trapping mutant of VE-PTP was immunoprecipitated for VE-PTP, and the precipitated material was analyzed in immunoblots for VE-cadherin (WB: VE-cadherin) or for VE-PTP (WB: VE-PTP). Bottom panel (WB: VE-cadherin total lysate) shows a VE-cadherin immunoblot of total cell lysates. (B) The cytoplasmic domain of VE-PTP is necessary for its T-cell–induced dissociation from VE-cadherin. Endothelioma cells generated from embryos of either WT mice (VE-PTP+/+) or from VE-PTPmut/mut mice lacking the cytoplasmic domain and the transmembrane domain of VE-PTP were incubated with or without T cells. Also, VE-PTP immunoprecipitates were analyzed in immunoblots for VE-cadherin (WB: VE-cadherin) or for VE-PTP (WB: VE-PTP). Bottom panel (WB: VE-cadherin total lysate) shows a VE-cadherin immunoblot of total cell lysates. (C) A model substrate of VE-PTP can dissociate VE-PTP from VE-cadherin. bEnd.5 cells were incubated without or with a cell-penetrating, phosphorylated Tie2 peptide (phospho-Tyr 992, Tat-pTyr) or the same nonphosphorylated peptide (Tat-Tyr), followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) Proposed signaling mechanism for the lymphocyte-induced dissociation of VE-PTP from VE-cadherin. Lymphocyte binding to VCAM-1 or stimulation by VEGF triggers the production of ROS via Rac1-mediated activation of NOX. This leads to activation of the redox-sensitive kinase Pyk2 that triggers, directly or indirectly, the phosphorylation of a VE-PTP substrate that, in turn, binds to VE-PTP. This binding may cause structural or conformational changes across the membrane that lead to detachment of the extracellular domain of VE-PTP from VE-cadherin. This facilitates phosphorylation of components or associated factors of the VE-cadherin-catenin complex, which participates in the destabilization of endothelial cell contacts.
Binding of a phosphorylated VE-PTP substrate induces VE-cadherin/VE-PTP dissociation. (A) VE-cadherin/VE-PTP association is severely reduced in CHO cells expressing a VE-PTP substrate-trapping (C/S) mutant compared with wild-type (WT) VE-PTP. CHO cells triple-transfected with VE-cadherin, VEGFR-2, and either the WT form or the C/S trapping mutant of VE-PTP was immunoprecipitated for VE-PTP, and the precipitated material was analyzed in immunoblots for VE-cadherin (WB: VE-cadherin) or for VE-PTP (WB: VE-PTP). Bottom panel (WB: VE-cadherin total lysate) shows a VE-cadherin immunoblot of total cell lysates. (B) The cytoplasmic domain of VE-PTP is necessary for its T-cell–induced dissociation from VE-cadherin. Endothelioma cells generated from embryos of either WT mice (VE-PTP+/+) or from VE-PTPmut/mut mice lacking the cytoplasmic domain and the transmembrane domain of VE-PTP were incubated with or without T cells. Also, VE-PTP immunoprecipitates were analyzed in immunoblots for VE-cadherin (WB: VE-cadherin) or for VE-PTP (WB: VE-PTP). Bottom panel (WB: VE-cadherin total lysate) shows a VE-cadherin immunoblot of total cell lysates. (C) A model substrate of VE-PTP can dissociate VE-PTP from VE-cadherin. bEnd.5 cells were incubated without or with a cell-penetrating, phosphorylated Tie2 peptide (phospho-Tyr 992, Tat-pTyr) or the same nonphosphorylated peptide (Tat-Tyr), followed by VE-PTP/VE-cadherin coimmunoprecipitation analysis. (D) Proposed signaling mechanism for the lymphocyte-induced dissociation of VE-PTP from VE-cadherin. Lymphocyte binding to VCAM-1 or stimulation by VEGF triggers the production of ROS via Rac1-mediated activation of NOX. This leads to activation of the redox-sensitive kinase Pyk2 that triggers, directly or indirectly, the phosphorylation of a VE-PTP substrate that, in turn, binds to VE-PTP. This binding may cause structural or conformational changes across the membrane that lead to detachment of the extracellular domain of VE-PTP from VE-cadherin. This facilitates phosphorylation of components or associated factors of the VE-cadherin-catenin complex, which participates in the destabilization of endothelial cell contacts.
On the basis of this idea, we hypothesized that the T-cell–induced signaling mechanism would trigger the binding of another substrate to VE-PTP, thereby detaching both proteins. Indeed, we found that the cytoplasmic domain of VE-PTP is necessary for the T-cell–induced dissociation of VE-PTP from VE-cadherin. This was shown by testing whether T cells can still trigger the dissociation of VE-PTP from VE-cadherin when binding to endothelioma cells that were established from VE-PTPmut/mut mice, lacking the entire cytoplasmic and transmembrane domains of VE-PTP.15 We found that the extracellular domain of the truncated VE-PTP still associated with VE-cadherin, but T cells were unable to trigger the dissociation of both proteins (Figure 7B).
To further test our hypothesis, we analyzed whether a model substrate of VE-PTP could interfere with the association of VE-PTP and VE-cadherin in endothelial cells. We have shown before that VE-PTP associates with the tyrosine kinase receptor Tie-2. Dissociating VE-PTP from Tie-2 with the help of antibodies leads to the phosphorylation of Y992 in the kinase activation loop of Tie-2.18 On the basis of this mechanism, we decided to use a peptide covering the activation loop of Tie-2 and carrying a phosphate group at Y992 as model substrate. To allow transfer of this model substrate into endothelial cells, we extended the peptide by a Tat peptide at its N terminus. For negative controls, we used the same peptide in its nonphosphorylated form. As shown in Figure 7C, incubation of bEnd.5 cells with 10 μM of the phosphorylated peptide for 5 minutes clearly reduced the efficiency of VE-cadherin coprecipitation with VE-PTP, whereas the nonphosphorylated control peptide had no such effect. We conclude that a substrate of VE-PTP can interfere with the association of VE-PTP and VE-cadherin in endothelial cells in a phosphorylation-dependent way. In combination with the fact that a trapping mutant of VE-PTP cannot associate with VE-cadherin and that the cytoplasmic tail of VE-PTP is necessary for the dissociation process, we suggest that the endothelial signaling mechanism that triggers the dissociation of VE-PTP from VE-cadherin mediates this process via stimulating the binding of a substrate to the intracellular phosphatase domain of VE-PTP.
Discussion
The cis-interaction of VE-PTP and VE-cadherin via extracellular domains supports the adhesive function of VE-cadherin and stabilizes endothelial contacts.17,20 We have recently shown that the detachment of VE-PTP from VE-cadherin is required for opening of endothelial junctions during leukocyte extravasation and for the induction of vascular permeability in vivo.21 Here, we have identified the adhesive endothelial receptor and the downstream signaling pathway whereby lymphocytes stimulate the dissociation of VE-PTP from VE-cadherin. The VCAM-1–triggered activation of Rac1 and NOX and the subsequent activation of Pyk2 were determined as central steps of this pathway, which turned out to be also required for the VEGF-stimulated dissociation of VE-PTP from VE-cadherin. Investigating the molecular mechanism that would detach both membrane proteins, we found that a model substrate of VE-PTP, when introduced into endothelial cells, could separate VE-PTP from VE-cadherin within minutes. Together with our finding that a trapping mutant of VE-PTP is strongly impaired in its association with VE-cadherin, our results suggest that the signaling mechanism we have identified may trigger a substrate of VE-PTP to interact with the phosphatase domain of VE-PTP, which leads to the physical detachment of both membrane proteins (Figure 7D).
VCAM-1 is a well-known endothelial adhesion receptor for lymphocytes and monocytes that is involved in the transmigration of these leukocytes through endothelial cell barriers.28,37 Besides its activities as an adhesion molecule, it also acts as a signaling receptor that affects endothelial cell contact integrity. VCAM-1 crosslinking was shown to destabilize endothelial cell contacts via a mechanism that required activation of Rac1 and the generation of ROS.29,31 Whether VCAM-1 crosslinking would also lead to the phosphorylation of Pyk2 was not directly investigated. Independent of VCAM-1, Rac1 activation, ROS production, and Pyk2 activation were found to support the destabilization of endothelial cell contacts.36 In the context of these reports, our results reveal the dissociation of VE-PTP from VE-cadherin as an important link that explains how the VCAM-1–triggered activation of Rac1, ROS production, and Pyk2 can destabilize endothelial junctions.
We could determine NOXs as the source for the production of ROS in the VCAM-1–driven signaling pathway because we applied the recently developed NOX inhibitor VAS2870. In contrast to other recently used inhibitors such as apocynin or DPI, VAS2870 does not have intrinsic antioxidant activity and does not interfere with other flavoproteins such as nitric oxide synthase and xanthine oxidase.38-40 We did not attempt to identify which NOX isoform is involved in the VCAM-1–stimulated signaling pathway. However, because only the activities of NOX1 and NOX2 are dependent on Rac1, it is highly likely that one of them is involved. Because NOX1 expression requires the angiogenic stimulation of endothelial cells by factors such as basic fibroblast growth factor or VEGF,41 it is more likely that the constitutively expressed NOX2 is involved in the process studied here.
It is remarkable that an inhibitor of the kinase Pyk2 (which also blocks the related kinase FAK) could block the dissociation of VE-PTP from VE-cadherin. Pyk2 was reported as being required for leukocyte-triggered phosphorylation of VE-cadherin.13 In agreement with this, Cain and colleagues could coprecipitate Pyk2 with VE-cadherin in resting endothelial cells, and this was enhanced on activation with TNF-α.42 Importantly, the Pyk2-related kinase FAK could not be detected in this report in VE-cadherin immunoprecipitates. In agreement with this result, we found that a FAK-specific inhibitor did not interfere with the dissociation of VE-PTP from VE-cadherin. Collectively, this observation suggests that the Pyk2 inhibitor we used, which also inhibits the kinase FAK, probably interfered with the VE-PTP/VE-cadherin dissociation via blocking Pyk2. This is not in conflict with other reports43,44 that clearly document a role for FAK and other kinases for the regulation of VE-cadherin function and the integrity of endothelial cell contacts. For instance, sphingosine-1-phosphate–induced strengthening of endothelial cell contacts has been reported to involve the recruitment of FAK to endothelial junctions.43 Furthermore, VEGF-induced destabilization of endothelial junctions was shown to require the recruitment of FAK to VE-cadherin, where it phosphorylated β-catenin.44 Other studies have reported Src and ERK as important kinases for leukocyte extravasation.13,35 Whereas we observed that inhibitors of these kinases did not interfere with the dissociation of VE-PTP from VE-cadherin, it is possible that they are important at later steps in the phosphorylation of adhesion-supporting structures at endothelial junctions.
Arguably, the most interesting step in the signaling mechanism that links VCAM-1 or the VEGF receptor with the dissociation of VE-PTP from VE-cadherin is the final step that physically triggers the dissociation. The concept for this final step was borne from the surprising observation that the C/S substrate-trapping mutant of VE-PTP was unable to associate with VE-cadherin in transfected CHO cells. At first glance, this mechanism may seem counterintuitive because a trapping mutant should rather bind more strongly to a substrate such as VE-cadherin. However, the robust association of VE-PTP and VE-cadherin is mediated via their extracellular domains, and both proteins are efficiently coprecipitated in the absence of their intracellular domains.17 In light of this, we suggest that the lack of the association of the C/S substrate-trapping mutant with VE-cadherin is the result of the potential obstruction of this interaction by other substrates that may irreversibly bind, even before the C/S VE-PTP mutant has the chance to interact with VE-cadherin. In agreement with our concept, we found that the cytoplasmic domain of VE-PTP was required in endothelial cells for the T-cell–induced dissociation of VE-PTP from VE-cadherin. In addition, we could show that a model substrate for VE-PTP, represented by the phosphorylated activation loop of Tie-2, was indeed sufficient to detach VE-PTP from VE-cadherin in intact endothelial cells. Of course, validating this concept for the physical detachment of VE-PTP from VE-cadherin requires identification of the substrate(s) that become tyrosine phosphorylated or otherwise empowered by the Rac1/NOX/Pyk2 signaling mechanism to bind to VE-PTP. However, our results can serve as proof of principle that shows that even a rather small substrate of VE-PTP (the Tat-activation loop construct has only an molecular weight of 3.2 kDa) is able to interfere with the robust association of VE-cadherin and VE-PTP, which is mediated by extracellular domains.
Because the binding of a cytosolic substrate to the intracellular part of VE-PTP counteracts the interaction of extracellular domains of VE-PTP with VE-cadherin, we propose that the intracellular binding transmits an allosteric effect on the extracellular part of VE-PTP across the plasma membrane. Indeed, other examples are known for such effects, although mainly restricted to homophilic or heterophilic dimeric proteins. Binding of talin and kindlins to the intracellular part of integrins changes their conformation,45 and oxidation-induced changes in the cytoplasmic domain of homophilic RPTP-α dimers lead to a change of extracellular conformation.46
It is still an open question as to which receptor mediates the neutrophil-stimulated dissociation of VE-PTP from VE-cadherin. Whereas the VCAM-1–binding integrin α4β1 was not found on circulating human neutrophils,47 it was found to be weakly expressed on circulating neutrophils, but relevant for neutrophil extravasation in the mouse.48,49 Therefore, we tested a role of VCAM-1 for mouse neutrophil–triggered VE-PTP/VE-cadherin dissociation. However, VCAM-1 antibodies had no blocking effect (unpublished observation). Also, soluble factors such as oxygen radicals, released by neutrophils, were ruled out to be relevant because catalase and superoxide dismutase had no effect.20 Thus, the receptor relevant for neutrophils in this context still needs to be identified.
In summary, we show here that lymphocyte binding to VCAM-1 or stimulation with VEGF triggers signaling processes in endothelial cells that share several central steps that lead via activation of Rac1, NOX, and the kinase Pyk2 to the dissociation of VE-PTP from VE-cadherin. In addition, we present evidence for a novel concept whereby a lymphocyte and a VEGF-driven signaling pathway may stimulate a VE-PTP substrate to bind to the intracellular phosphatase domain of VE-PTP and thereby dissociate the 2 membrane proteins. As destabilization of this complex is an essential step during the opening of endothelial junctions, further studies of this mechanism may open new opportunities for interfering with inflammatory disorders and pathological vascular permeability.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Carlo Laudanna (University of Verona) for generously providing the Tat-Rac1 DNA constructs and are grateful to Astrid Nottebaum and Stefan Butz (Max Planck Institute, Münster, Germany) for carefully reading the manuscript.
This work was supported by funds from the Deutsche Forschungsgemeinschaft (SFB629) and from the Max Planck Society.
Authorship
Contribution: M.V. performed and designed the experiments and wrote the manuscript; and D.V. initiated the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dietmar Vestweber, Max Planck Institute for Molecular Biomedicine, Röntgenstr. 20, D-48149 Münster, Germany; e-mail: vestweb@mpi-muenster.mpg.de.




![Figure 5. Pyk2 activity regulates VE-cadherin/VE-PTP dissociation. (A) Pyk2 inhibition prevents VE-cadherin/VE-PTP dissociation. TNF-α–stimulated bEnd.5 cells were preincubated with the indicated concentrations of the Pyk2/FAK inhibitor PF431396, followed by the incubation with or without T cells and subsequent VE-PTP/VE-cadherin coimmunoprecipitation analysis. (B) FAK activity is not required for VE-cadherin/VE-PTP dissociation. Analysis done is analogous to panel A, comparing the inhibitory activity of the dual-specificity Pyk2/FAK inhibitor PF431396 (5 μM) and the monospecific FAK inhibitor PF573228 (5 μM). (C) Pyk2 acts downstream of NOX activation. TNF-α–stimulated bEnd.5 cells were preincubated with the NOX inhibitor VAS2870 (10 μM) and were subsequently stimulated with or without anti–VCAM-1 mAb-loaded beads (VCAM XL), followed by immunoprecipitation of Pyk2 and immunoblotting with a general antiphosphotyrosine mAb (WB: pTyr), antibodies against phosphorylated tyrosine 402 of Pyk2 (WB: pPyk2 [Tyr402]), or antibodies against Pyk2 (WB: Pyk2). Equivalent aliquots of total cell lysates were analyzed by immunoblotting for Pyk2 (WB: Pyk2 total lysate).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/14/10.1182_blood-2013-04-499228/4/m_2512f5.jpeg?Expires=1767810146&Signature=tECn6XQEQ26B02Wja~QZ~1HwZH5S2EXkNNp1czit6-tD4UuJPJy2-Zpmtz6DRAZ6ovyoicj6vxBJr24TzpKgEsLCZk9Q8FomMCBQCFa4wIW4kfUTeTDdkt7ZmH509NAPdoBXrIt~dYzz5PatO2OMyjx-PRqVFdt80lNlEVTLPrgc4QTzZ9qzm2q8BQALN15Fpwkq3bwjWKZf3AMipo6xwS81PdMuNh3b-KqdwbLyyokBsYDzfYwil-~tH3-HMl-EmhBEVygr3JBujzJChmPyKiTcRJaC26Zb1eBLRIKk1qQThA1H4PBedkZREjVCcmk9bl23ydwWgTKBaPkHcgI~Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal