To the editor:
Montes-Moreno et al recently reported the usefulness of SPIB as a novel immunohistochemical marker for supporting the diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN).1 The article highlights the well-recognized difficulty in approaching the diagnosis of BPDCN, in which transformed plasmacytoid dendritic cells (PDCs) frequently show loss and/or de novo expression of antigens that may lead to misdiagnosis.2,3 In the study of Montes-Moreno,1 all 44 cases of BPDCN were positive for SPIB, whereas the classical mimickers, including acute myeloid leukemia (AML) and precursor acute lymphoblastic leukemia (ALL), were regularly negative. Nevertheless, SPIB showed reactivity with normal B-cells and were labeled neoplastic cells of a large proportion (58%) of diffuse large B-cell lymphomas and of a minority (18%) of peripheral T-cell lymphomas.
Here, we would like to integrate data from the study of Montes-Moreno1 by proposing an anti-BDCA-2 (CD303) antibody as an additional tool in the BPDCN diagnostic panel. BDCA-2 is a type II C-type lectin receptor selectively expressed on PDCs, where it is involved in antigen capture and in regulation of the production of interferon type I. Dzionek et al4 originally described the specific reactivity with normal PDCs of the anti-BDCA-2 mouse clone AC144 applied on frozen tissue sections; subsequently, Jaye et al,5 developed a rabbit polyclonal anti-BDCA-2 antibody in their laboratory that stained normal PDCs and BPDCN tumor cells on frozen and formalin-fixed paraffin-embedded tissue sections. Using a new anti-BDCA-2 mouse monoclonal antibody reactive on formalin-fixed paraffin-embedded tissue sections (clone 124B3.13; Dendritics, Lyon, France), we stained a large series of normal and reactive lymphoid tissues and found that this reagent is an excellent marker for human PDCs, being selectively and strongly expressed on these cells (Figure 1). Furthermore, the antibody stained neoplastic cells in 19 of 21 cases of BPDCN, whereas it was regularly negative in all samples from AML (n = 55, all FAB subtypes), ALL (n = 15, 10 B-cell and 5 T-cell type), diffuse large B-cell lymphomas (n = 35), and peripheral T-cell lymphoma (n = 25). These results support data from previous studies that used the same anti-BDCA-2 clone on a limited number of cases,6-8 thus indicating that this reagent has a very high specificity (100%), sensitivity (90.5%), and positive predictive value (100%) for BPDCN. Remarkably, the high level of performance of the clone 124B3.13 is unique among the large set of PDC markers commonly used for BPDCN diagnosis, such as CD123, TCL1, BCL11a, CD2AP, as well as SPIB.1-3,9 Moreover, our findings complement previously published data obtained by flow cytometry using the AC144 clone,10 that demonstrated that positivity for BDCA-2 has the highest diagnostic score within a panel of markers used for BPDCN identification and confirmed that BPDCN does not cross the boundary with other hematopoietic neoplasms.10
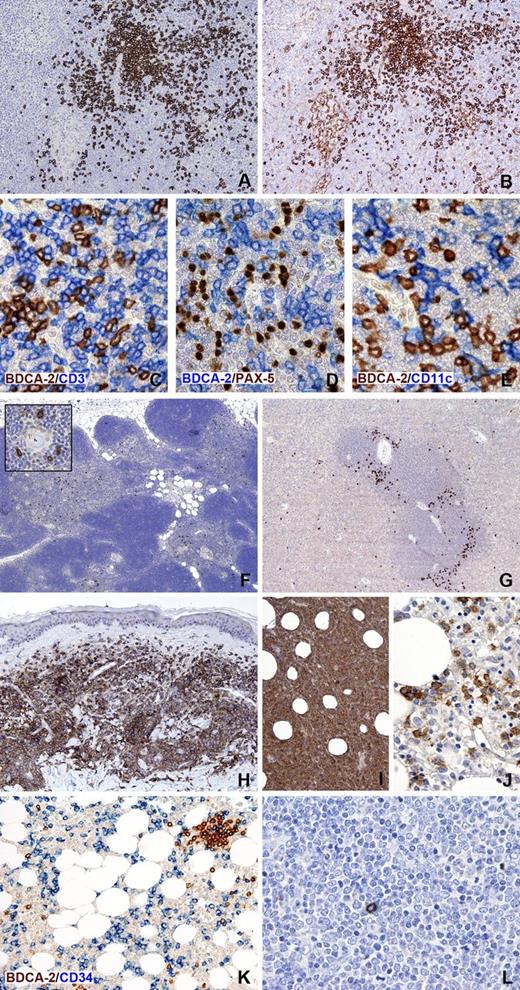
Immunohistochemical expression of BDCA-2 in normal lymphoid tissues and hematopoietic neoplasms. BDCA-2 immunostaining of a reactive lymph node (A) identifies aggregates and dispersed PDCs; in contrast, note that anti-CD123 applied on a serial section labels not only PDCs but also histiocytes and high endothelial venule endothelium (B). BDCA-2 is selectively expressed on PDCs and not on T-cells, B-cells, and macrophages/myeloid dendritic cells, as shown by double immunostainings, respectively, for BDCA-2 and CD3 (C), PAX-5 (D), and CD11c (E). In the thymus, BDCA-2 highlights scattered PDCs located in the medulla (F), where they frequently surround Hassall’s corpuscles (insert). In the spleen, BDCA-2-positive PDCs are particularly located at the periphery of the white pulp (G). Neoplastic PDCs show strong positivity for BDCA-2, as illustrated in representative cases of BPDCN with cutaneous (H) and bone marrow involvement (I-J). BDCA-2 can be very helpful in detecting minimal tumor cell infiltrate in the bone marrow (J).It must be noted, however, that BDCA-2 reactivity in this tissue can be variable, likely due to antigen degradation consequently from decalcification. BDCA-2 is totally negative on tumor cells in 2 representative samples of AML (K) and ALL (L), in which rare reactive PDCs are also detected. In the AML case (M2 in FAB classification) myeloid blasts are highlighted by anti-CD34. Single immunostaining for BDCA-2 was performed after heat-based antigen retrival in ethylenediaminetetraacetic acid buffer, pH 8.0; reactivity was revealed using the NovoLink Polymer kit (Leica Microsystems, Newcastle upon Tyne, United Kingdom) followed by diaminobenzidine as brown chromogen. For double immunostainings (C-E and K), the blue reaction visualizing the second antibody was obtained using the Mach 4-AP kit followed by Ferangi Blue as chromogen (Biocare Medical, Concord, CA). Images were obtained with the BX60 microscope, DP-70 digital camera, and image processing software (Olympus, Hamburg, Germany). Original magnifications, ×40 (F), ×100 (G-I), ×200 (A, B, K), and ×400 (C-E, inset in F, and J and L).
Immunohistochemical expression of BDCA-2 in normal lymphoid tissues and hematopoietic neoplasms. BDCA-2 immunostaining of a reactive lymph node (A) identifies aggregates and dispersed PDCs; in contrast, note that anti-CD123 applied on a serial section labels not only PDCs but also histiocytes and high endothelial venule endothelium (B). BDCA-2 is selectively expressed on PDCs and not on T-cells, B-cells, and macrophages/myeloid dendritic cells, as shown by double immunostainings, respectively, for BDCA-2 and CD3 (C), PAX-5 (D), and CD11c (E). In the thymus, BDCA-2 highlights scattered PDCs located in the medulla (F), where they frequently surround Hassall’s corpuscles (insert). In the spleen, BDCA-2-positive PDCs are particularly located at the periphery of the white pulp (G). Neoplastic PDCs show strong positivity for BDCA-2, as illustrated in representative cases of BPDCN with cutaneous (H) and bone marrow involvement (I-J). BDCA-2 can be very helpful in detecting minimal tumor cell infiltrate in the bone marrow (J).It must be noted, however, that BDCA-2 reactivity in this tissue can be variable, likely due to antigen degradation consequently from decalcification. BDCA-2 is totally negative on tumor cells in 2 representative samples of AML (K) and ALL (L), in which rare reactive PDCs are also detected. In the AML case (M2 in FAB classification) myeloid blasts are highlighted by anti-CD34. Single immunostaining for BDCA-2 was performed after heat-based antigen retrival in ethylenediaminetetraacetic acid buffer, pH 8.0; reactivity was revealed using the NovoLink Polymer kit (Leica Microsystems, Newcastle upon Tyne, United Kingdom) followed by diaminobenzidine as brown chromogen. For double immunostainings (C-E and K), the blue reaction visualizing the second antibody was obtained using the Mach 4-AP kit followed by Ferangi Blue as chromogen (Biocare Medical, Concord, CA). Images were obtained with the BX60 microscope, DP-70 digital camera, and image processing software (Olympus, Hamburg, Germany). Original magnifications, ×40 (F), ×100 (G-I), ×200 (A, B, K), and ×400 (C-E, inset in F, and J and L).
Montes-Moreno et al1 concluded that SPIB expression supports the diagnosis of BPDCN in the appropriate clinicopathological setting, but needs to be evaluated in association with other immunohistochemical markers. Here, we propose that anti-BDCA-2 clone 124B3.13 should be included as an additional tool in the standard diagnostic panel for BPDCN.
Authorship
Acknowledgments: This work was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (grant 20104HBZ8E to F.F.). L.B. was supported by Fondazione Angelo Nocivelli. Approval was obtained from the Spedali Civili-University of Brescia institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Contributions: L.B. and S.L. interpreted the data; S.L. performed the experiments; W.V., S.F., and F.F. collected the data; W.V. and S.F. analyzed the data; F.F. supervised the study, reviewed the data, and revised the manuscript for intellectual content; and L.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Facchetti, Department of Molecular and Translational Medicine, Anatomic Pathology Section, University of Brescia, Spedali Civili of Brescia, 25123, Brescia, Italy; e-mail: facchett@med.unibs.it.
References
Author notes
L.B. and S.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal