In this issue of Blood, Casucci et al present an elegant study that describes a potential new target for adoptive cell transfer (ACT), in this case CD44 splice variant 6 (CD44v6), and detail why it may be a good target for ACT and how to manage expected off-tumor/on-target toxicities.1
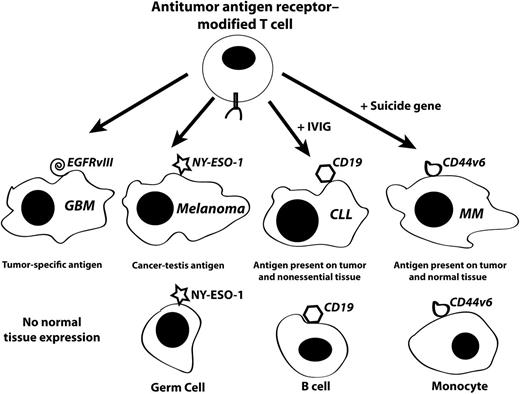
Target antigen types for adoptive cell therapy. T cells can be genetically modified with tumor-targeting receptors (either T-cell receptors or CARs) and administered to patients in adoptive cell therapy trials. The types of tumors and tumor antigens targeted by these modified T cells can have no or significant off-tumor/on-target toxicity dependent on antigen expression. For tumor-specific antigens such as EGFRvIII in glioblastoma (GBM), there will likely be no toxicity due to the lack on antigen expression in normal tissues. Some (but not all) CT antigens have gene expression patterns limited to cancer and the non–MHC-bearing germ cells in the testis (eg, NY-ESO-1 in melanoma). It may be clinically acceptable to target tumor antigens that are expressed in malignancies and nonessential tissues such as the example of CD19, which is expressed in chronic lymphocytic leukemia (CLL) but also in normal B cells. B-cell depletion can be managed by IV immunoglobulin (IVIG) administration. The majority of tumor antigens follow the pattern investigated by Casucci et al, where the CD44v6 antigen is expressed in the tumor (eg, MM) as well as in normal tissues. As a potential method to manage expected monocytopenia, the investigators suggest that a suicide gene could be used to eliminate T cells after the antitumor response is complete.
Target antigen types for adoptive cell therapy. T cells can be genetically modified with tumor-targeting receptors (either T-cell receptors or CARs) and administered to patients in adoptive cell therapy trials. The types of tumors and tumor antigens targeted by these modified T cells can have no or significant off-tumor/on-target toxicity dependent on antigen expression. For tumor-specific antigens such as EGFRvIII in glioblastoma (GBM), there will likely be no toxicity due to the lack on antigen expression in normal tissues. Some (but not all) CT antigens have gene expression patterns limited to cancer and the non–MHC-bearing germ cells in the testis (eg, NY-ESO-1 in melanoma). It may be clinically acceptable to target tumor antigens that are expressed in malignancies and nonessential tissues such as the example of CD19, which is expressed in chronic lymphocytic leukemia (CLL) but also in normal B cells. B-cell depletion can be managed by IV immunoglobulin (IVIG) administration. The majority of tumor antigens follow the pattern investigated by Casucci et al, where the CD44v6 antigen is expressed in the tumor (eg, MM) as well as in normal tissues. As a potential method to manage expected monocytopenia, the investigators suggest that a suicide gene could be used to eliminate T cells after the antitumor response is complete.
There has been an explosion of interest in field of adoptive cell transfer due to the recent clinical success reported by several groups in the treatment of B cell malignancies.2-4 Durable clinical responses have been documented in several patients, including those heavily pretreated with standard chemotherapy, using T cells genetically engineered to express chimeric antigen receptors (CARs) directed to the CD19 antigen.
Choosing target antigens for adoptive cell therapy is tricky (see figure). Ideally, a tumor antigen would be tumor specific; that is, not expressed in any normal tissue. Although chromosomal translocations are well documented in a variety of malignancies, those rearrangements that reproducibly result in the exact same novel protein sequence are rare. An example of this type of tumor antigen is one of the rearrangements of the epidermal growth factor receptor (EGRF variant III, or EGFRvIII), which is commonly found in glioblastoma as well as some other cancers.5 Viral-associated cancers are also in this category (eg, human papillomavirus 16/17–associated cervical cancer). Some cancers with large numbers of environmentally induced mutations (ie, lung cancer and melanoma) likely have patient-specific mutations that induce highly specific immune responses.6
Unfortunately, the majority of tumors do not express specific mutations with common genetic alterations that can be targeted by antitumor antigen receptor–modified T cells. A potentially attractive alternative to the cancer-specific antigens may be a class of antigens known as cancer-testis (CT) antigens.7 The CT antigens (also called cancer-germ line antigens) are generally (but not exclusively) expressed in the testis and many tumor types. In the case of T-cell receptor–modified T cells, the testis is not a target for immunologic attack because the CT-expressing germ cells do not express major histocompatibility complex molecules. An example of a CT antigen that has been successfully targeted in adoptive cell therapy clinical trials is the NY-ESO-1 protein (gene name CTAG1B).8 The CD19 antigen is an example of a tumor antigen that is expressed in tumors and nonessential tissues. In the successful clinical trials describing anti-CD19 CAR therapies, prolonged B-cell depletion was observed in several patients, and this necessitated the use of IVIG therapy. The majority of tumor antigens are in a class of proteins that are overexpressed in tumors but also have normal tissue expression. The CD44v6 tumor antigen is an example of this class of tumor antigen.
Casucci et al demonstrated that CD44v6 was expressed on the majority of acute myeloid leukemia (AML) and multiple myeloma (MM) samples analyzed. Furthermore their data suggest that CD44v6 may be necessary for the establishment of tumors because when the gene was specifically knocked out using short interfering RNA, tumor engraftment in immunocompromised mice was significantly reduced. To target the CD44v6 antigen, the authors developed a CAR based on the anti-CD44v6 monoclonal antibody bivatuzumab and linked this to T-cell signaling domains from the CD28 and CD3ζ proteins. Using retroviral vectors to transfer the CAR into primary human T cells yielded T cells that displayed the appropriate in vitro anti-CD44v6 effector functions and actively inhibited AML and MM tumor formation in xenograft models. Many investigators would publish their data at this point, but for those investigators serious about human applications, this is insufficient.
Normal tissue expression of CD44v6 was investigated at the RNA and protein levels and found to be present at easily detectable levels in keratinocytes and circulating monocytes. Although keratinocytes were not recognized by anti-CD44v6 CAR-engineered T cells, monocytes were and monocytopenia was observed in animal models. This type of off-tumor/on-target toxicity will be an unavoidable consequence for many adoptive cell therapy approaches using receptor-modified T cells, and whereas transient monocytopenia may be manageable, long-term monocytopenia is not. Therefore, the investigators added a suicide gene to their gene transfer system (specifically the nonimmunogenic and quick-acting iCasp9 system) and were able to show rapid elimination of gene-engineered T cells in in vivo models.
The report by Casucci et al is an excellent example of the identification of a potentially valuable tumor antigen for adoptive cell therapy that combines a target that may be essential for tumorigenesis in multiple cancers, with a detailed plan for clinical development based on the knowledge of potential toxicities and potential means to limit those toxicities.
Conflict-of-interest disclosure: The author declares no competing financial interests.