Abstract
Cardiac iron removal is relatively slow and patients with cardiac siderosis can take years to normalize cardiac T2* to >20 ms. Prospective comparison of iron chelators are mostly limited to studies of 1-yr duration. CORDELIA is a large randomized trial comparing deferasirox (DFX) with deferoxamine (DFO) in patients with β-thalassemia major (TM), which demonstrated the non-inferiority of DFX vs DFO for cardiac iron removal at 1 yr, with a trend for superiority of DFX (P=0.057). This 1-yr extension was planned to collect additional data on efficacy and safety of DFX and DFO in patients with cardiac siderosis when treated for up to 2 yr.
Study design has been reported previously (Pennell Blood 2012; abst 2124). Patients enrolled had cardiovascular magnetic resonance-measured cardiac T2* 6–20 ms, left ventricular ejection fraction (LVEF) ≥56%, and R2-magnetic resonance imaging liver iron concentration (LIC) ≥3 mg Fe/g dw. Patients completing 1 yr were eligible to continue on DFX or DFO as assigned, or to switch treatment on entering the extension if judged by the investigator to be of therapeutic benefit. Target doses were an intensified DFO regimen of 50–60 mg/kg/d sc for 8–12 h, 5–7 d/wk, or DFX at 40 mg/kg/d. Efficacy is reported for changes from core baseline (BL). Safety was monitored continuously. Results for patients continuing with DFX or DFO are reported here.
In total, 160/197 patients completed 1 yr; 74 patients continued into the extension on DFX (mean age 20.1 ± 6.9 yr; 59.5% male) and 29 patients on DFO (17.0 ± 5.4 yr; 58.6%). At core BL, 29.7% DFX patients had cardiac T2* <10 ms, with geometric mean (Gmean) T2* 11.6 ms (CV 30.9) and 37.9% DFO patients had T2* <10 ms, with T2* 10.8 ms (CV 29.8). Mean LIC was 30.6 ± 18.0 for DFX and 36.8 ± 15.6 mg Fe/g dw for DFO. Of the DFX patients, 65 completed 2 yr (discontinuation due to 1 adverse event [AE]; 4 unsatisfactory therapeutic effect; 2 consent withdrawal; 1 lost to follow-up and 1 death); 24 DFO patients completed (1 abnormal test procedure result; 1 unsatisfactory therapeutic effect; 2 consent withdrawal; 1 lost to follow-up).
Mean dose over 2 yr was 35.5 ± 6.3 mg/kg/d DFX, and 57.0 ± 13.3 mg/kg/d DFO for 5 d/wk (40.7 ± 9.5 mg/kg/d for 7 d/wk). Overall, 99.1 and 98.1% of the planned DFX and DFO dose was taken, respectively.
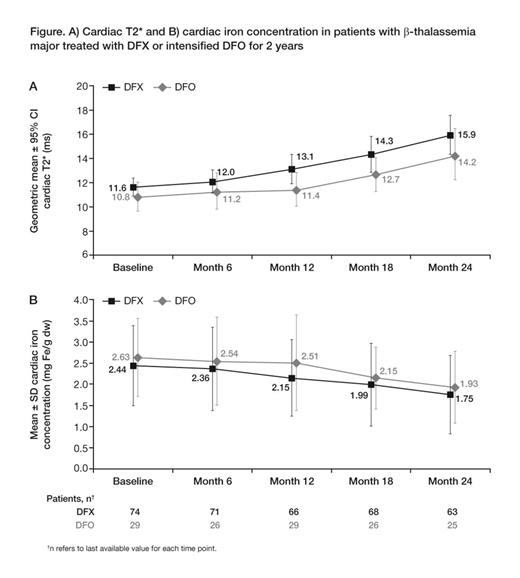
At month 24, cardiac T2* increased to 15.9 ms (CV 43.4) in DFX patients (Gmean ratio of month 24/BL 1.38 [95% CI 1.28, 1.49]) and to 14.2 ms (CV 37.3) in DFO patients (ratio 1.33 [1.13, 1.55]), indicating a 38 and 33% improvement, respectively (Fig A). Estimation of cardiac iron concentration (CIC) showed decreases during 2-yr treatment with both DFX and DFO (Fig B). Mean LVEF was stable and remained within normal limits with both DFX (change at month 24: +0.6 ± 4.7%) and DFO (–0.6 ± 5.0%). LIC decreased to 14.4 ± 16.6 mg Fe/g dw at month 24 in DFX patients (–15.7 ± 15.2 mg Fe/g dw; 95% CI –19.7, –11.8) and to 11.0 ± 12.1 mg Fe/g dw in DFO patients (–26.0 ± 14.9 mg Fe/g dw; 95% CI –32.3, –19.7).
Over 2 yr, most common AEs (>10%) regardless of causality for DFX were upper respiratory tract infection (17.8%), diarrhea (13.7%), influenza (15.1%), nasopharyngitis (16.4%), increased alanine aminotransferase (ALT; 12.3%); increased blood creatinine (12.3%), increased aspartate aminotransferase (11.0%) and back pain (11.0%); and for DFO, pyrexia (13.8%), upper abdominal pain (10.3%), increased platelet count (20.7%) and urticaria (10.3%). Serious AEs with suspected relationship to study drug were reported in 6.8% of DFX and 6.9% of DFO patients. A DFX patient died due to cardiac arrest which was not considered related to study drug.
Serum creatinine increases >33% from BL and > upper limit of normal (ULN) at 2 consecutive values occurred in 2.7% of DFX and 3.4% of DFO patients. Frequency of ALT elevations >5 x ULN and 2 x BL were comparable between groups.
Cardiac T2* increased substantially during 2-yr treatment with DFX or DFO. Improvement with DFX was comparable with DFO, although DFO patient numbers were low. The magnitude of cardiac T2* improvement with DFX was consistent with previous long-term studies (Pennell Blood 2010). Mean LVEF was stable and remained within normal limits in both groups. LIC continued to decrease from very high BL levels with DFX and DFO. The reduction in CIC in the extension was similar or possibly greater than in the core, which might relate to the falling LIC. Long-term safety profiles of DFX and DFO were consistent with previous reports.
Pennell:Shire: Consultancy, Honoraria; ApoPharma: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Porter:Celgene: Consultancy; Shire: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Piga:Novartis: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Wegener:Novartis: Employment. Habr:Novartis: Employment. Shen:Novartis: Employment. Aydinok:Shire: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal