Abstract
The median survival (OS) of patients with primary myelofibrosis (PMF; 6.5y) is significantly shortened compared to reference population (Cervantes et al, JCO 2012; 30:2981). OS is predicted by the four risk categories of IPSS, Dynamic-IPSS and DIPSS-plus score, nevertheless pts heterogeneity persists within these categories, necessitating improved risk stratification. We recently reported that pts harboring mutations in any one of the prognostically relevant ASXL1, EZH2, IDH1/2 and SRSF2 genes constitute a IPSS-and DIPPS-plus independent Molecular High Risk category (MHR+) characterized by significant reduction of OS and leukemia free survival (LFS) (Vannucchi et al, Leukemia 2013). The aim of this work was to analyze the impact of the number of prognostically relevant mutated genes on OS and LFS in PMF.
Two independent cohorts are included, a “test cohort” from Europe, analyzed at diagnosis, and a “validation cohort” from Mayo Clinic, analyzed at any time after diagnosis. Mutation analysis was performed in DNA from whole blood or granulocytes using RTQ-PCR, HRM and direct sequencing; all mutations were confirmed at least twice. The prognostic value of the molecular variables with regard to OS and LFS was analyzed by Cox regression.
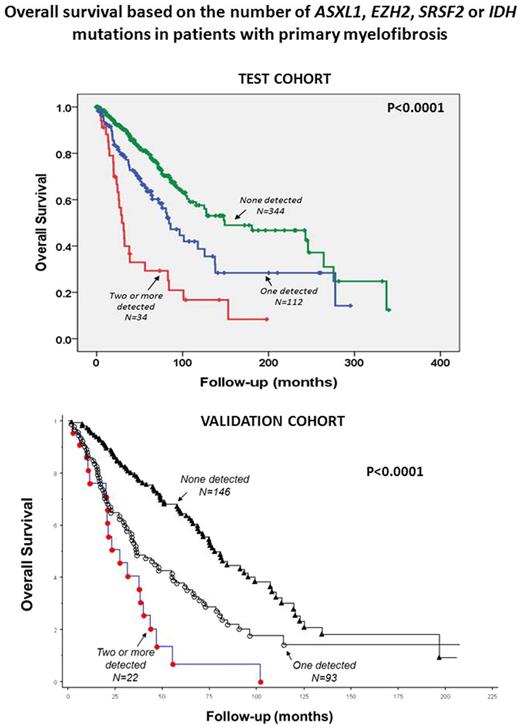
It included 490 pts (median age 61y; males = 301) risk stratified by IPSS into high (n=74, 15%), intermediate-2 (n=93, 19% ), intermediate-1 (n=147, 30%) and low (n=176, 36%). The median follow-up was 3.63y (95% CI, 0.06-28.33); 161 pts died (33.0%), of whom 76 (15.6%) had progressed to acute leukemia after a median of 3.4y (0.04-28.3) from diagnosis. One hundred forty-six pts (29.8%) presented at least one of the four aforementioned mutated genes and were classified as MHR+. The OS of MHR+ pts was significantly reduced compared to patients with no mutations (n=344): 80.7 vs 148.9 mo (HR 2.2, CI95% 1.6-3.03). One hundred twelve (22.8%) pts had 1 mutation and 34 (6.9%) had 2 or more mutations. In univariate analysis presence of 2 or more mutated genes was significantly more detrimental for OS (29.5mo; HR 4.12, IC95% 2.6-6.4) than having 1 mutated gene (84.2mo; HR 1.8, IC95% 1.2-2.5) compared with having no mutations (148.9mo). Multivariate analysis results adjusted for IPSS indicated that having two or more mutations was an independent prognostic factor for OS (HR 2.9, 95% CI 1.8-4.5). Notably, the prognostic relevance of harboring two or more mutations involved both lower (low and intermediate-1; HR 1.87, 95% CI 1.3-2.6) and higher (intermediate-2 and high; HR 1.6, 95% CI 1.2-2.1) categories of IPSS. Also LFS resulted significantly shorter (129mo; HR 3.1, 95%CI 1.9-4.8) in MHR+ pts compared with pts with no adverse mutations (323mo). Having 2 or more mutations correlated with greater reduction of LFS (79.6mo; HR 7.02, CI95% 3.9-12.6) than having one mutation only (133.9mo; HR 2.2, IC95% 1.3-3.7) as compared with no mutations (323.2mo). Multivariate analysis showed that IPSS high risk category (HR 4.5; 95% CI 2.3-8.8) and two or more mutated genes (HR 5.3; 95% CI 2.9-9.8) predicted independently for a significant reduction in LFS. The negative impact on LFS of having 2 or more mutated genes compared to one or no mutations was maintained in the lower (HR 6.4, 95% CI 2.6-15.2) and higher (HR 5.5, 95% CI 2.3-12.8) risk categories.
Validation cohort included 262 patients (median age 64 years; males = 167) risk stratified by DIPSS-plus into high (n=89, 34%), intermediate-2 (n=92, 35%), intermediate-1 (n=46, 18%) and low (n=34, 13%). One hundred forty-six pts (56%) displayed none of the four aforementioned mutations, 93 (36%) harbored one mutation whereas 23 (9%) harbored two or more mutations. In univariate analysis, having two or more mutations (HR 3.7; 95% CI 2.2-6.1) was significantly more detrimental for OS than having no mutations, which is more favorable than having one mutation (HR 1.9; 95% CI 1.4-2.7). When adjusted for DIPSS-plus, the presence of two or more mutations retained its significance (HR 2.1; 95% CI 1.3-3.6) and outperformed ASXL1 mutation alone in its prognostic relevance
Overall, these results show that the number of prognostically relevant mutated genes correlate with OS and LFS in pts with PMF, suggesting that screening for these mutations might help to improve risk stratification.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal