Abstract
Sepsis and septic shock are leading causes of ICU mortality. They are characterized by excessive host inflammation, upregulation of procoagulant proteins and depletion of natural anticoagulants. Therapeutic apheresis has the potential to improve survival in sepsis by removing injurious elements and inflammatory cytokines and restoring deficient plasma proteins. The objective of our systematic review was to evaluate the efficacy and safety of apheresis in patients with sepsis or septic shock.
We searched PubMed, EMBASE, and CENTRAL (from inception to February 2013), the International Clinical Trials Registry Platform, relevant conference proceedings and bibliographies of pertinent reviews and included clinical trials. Two reviewers independently identified randomized controlled trials of patients diagnosed with sepsis, severe sepsis, septic shock or disseminated intravascular coagulation due to infection who received plasmapheresis, plasma exchange, or plasma filtration compared to placebo or usual care. Two reviewers independently extracted trial-level data including population characteristics, interventions, outcomes, and funding sources. We assessed risk of bias using the Cochrane risk of bias tool. Our primary outcome was all-cause mortality reported at the longest follow-up. Secondary outcomes were hospital and ICU lengths of stay, and reported adverse events. We expressed summary effect measures as odds ratios (OR) with 95% confidence intervals (CI). Random effect models using the Mantel-Haenszel method were used for pooled analyses.
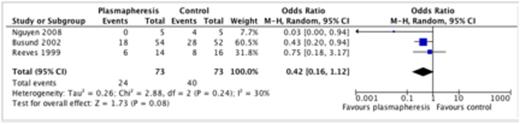
We identified 1771 potential citations of which 3 trials (144 patients) met inclusion criteria. The mean age of patients ranged from 38 to 53 years in the two adult trials and 1 to 18 years in the single pediatric trial. The mean APACHE score was 25.2 (APACHE II) in one study and 54.9 (APACHE III) in the other study reporting illness severity scores. All 3 studies were adjudicated to be unclear or high risk of bias. We observed that the use of apheresis was not associated with a significant reduction in all cause mortality (OR 0.42, 95% CI 0.16 - 1.12, I2=30%) (see Figure). In a subgroup analysis of studies including children exclusively, we observed that apheresis was associated with a significant reduction in mortality (OR 0.03, 95% CI 0.00 – 0.94). None of the included studies reported ICU or hospital length of stay. Only one study reported adverse events associated with apheresis including 6 episodes of hypotension and one allergic reaction to fresh frozen plasma. Central-venous catheter related complications were not reported.
Mortality and apheresis in sepsis and septic shock
In patients with sepsis or septic shock, apheresis is not associated a significant reduction in all cause mortality. There is currently insufficient evidence to recommend apheresis as an adjunctive therapy in patients with sepsis or septic shock. Rigorous randomized controlled trials powered to detect differences in patient-centered, clinically relevant outcomes are required to evaluate the impact of apheresis in this patient population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal