Abstract
The current treatment paradigm for acute myeloid leukemia (AML) is remission induction chemotherapy, followed by either consolidation chemotherapy or allogeneic stem cell transplantation. As most patients diagnosed with AML are in their sixth or seventh decade of life, many are not candidates for standard remission induction chemotherapy because of the risk of toxicities, such as profound myelosuppression, life-threatening infections, and cardiotoxicity. The development of new effective and safe treatments for AML is therefore needed. The “ideal” therapy should specifically target AML tumor cells with no side-effect on normal cells. Since CD117 (c-Kit) is a transmembrane receptor on tumor cells surface and expresses in 70% cases of AML, it is a potential molecule for developing new targeted therapy.
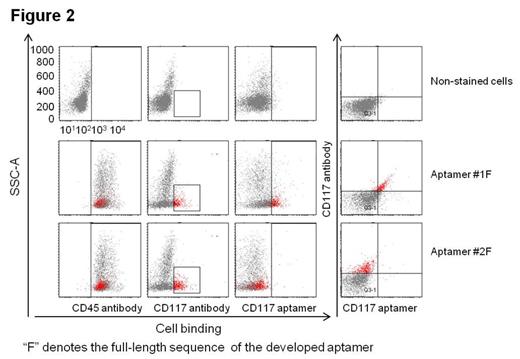
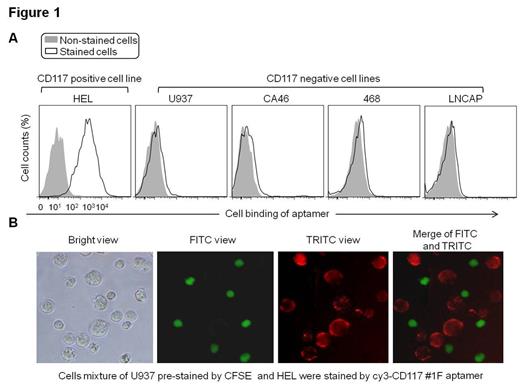
Aptamers are single-stranded oligonucleotides (DNA or RNA), which have the ability to specifically bind to their targets with high affinity. As a “chemical antibody”, aptamers can be chemically synthesized, easily conjugated with therapeutic drugs, and more importantly, less or not immunogenic. In this study, we developed single strand DNA (ssDNA) aptamer specific for CD117 by using a unique hybrid SELEX approach with both cell-selection and protein-enrichment. When sequencing the enriched ssDNA library with million reads, one dominant sequence had over 80% copies of total sequence reads. Cell binding analysis of the aptamer by flow cytometry and fluorescent microscopy (figure 1) demonstrated that, the aptamer molecule specifically bound to CD117-expressing AML cells, but did not react to CD117-negative control cells including Histiocytic lymphoma Cell line: U937, Burkitt's lymphoma cell line: CA46, Breast adenocarcinoma cell line: 468 and human prostate carcinoma cell line: LNCap. In addition, the presence of aptamers inhibited cell binding anti-CD117 antibody, indicating that aptamer targeted CD117 receptor on leukemic cells. Notably, this aptamer specifically targeted CD117-positve AML cells of clinical specimens with an identical staining pattern to that observed with anti-CD117 antibody (figure 2), indicating potential for in clinical use. For targeted therapy, an aptamer -drug-conjugate was formulated by chemical conjugation of ssDNA CD117 aptamer (Apt) to chemotherapeutic drug, Methotrexate (MTX). For treatment study, cell mixture of leukemic cells HEL (CD117+) and U937 (CD117-), which showed the same sensitivity to free MTX, were used (figure 3A). Exposure of cells to the Apt-MTX revealed that the formed aptamer-drug-conjugate specifically killed CD117 positive cells, but had no effect on the growth of the off-target cells in the same cultures. For therapeutic effect, Apt-MTX killed 60% of positive cells at as low as 10nM final concentration (figure 3B). In contrast, under the same condition, free MTX had no effect on cell growth (figure 3A). Our study demonstrated that the aptamer-drug-conjugate could be a new targeted therapeutics in addition to current antibody-drug-conjugate.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal