Abstract
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal myeloid disorders characterized by marrow failure and variable risk for acute leukemia transformation. The revised International prognostic scoring system (R-IPSS) biologically defines 5 risk disease categories and assists in logistic outcome prediction and treatment algorithm. For those patients (pts) with high risk MDS, treatment with hypomethylating agents (HMAs) such as azacitidine (AZA) and decitabine (DAC) is associated with prolonged survival. However primary and secondary treatment failures are common phenomenon and treatment outcomes after failure are dismal (Prebet T et al. JCO. 2011; Prebet T. Haematologica.2013). Mechanisms of loss of response to azanucleosides are largely unknown. Cytogenetic evolution (CE) defined as the acquisition of additional clones or karyotypic abnormalities in pts with preexisting normal or aberrant karyotype, during disease course or after treatment, is observed in about 30% of MDS patients, and is linked to genomic instability resulting in adverse outcomes. We investigated risk factors for CE in MDS pts treated with HMAs and its impact of OS.
From 2000-2012, 13/124 pts (16%) (Median age 59 years; range 54-80) with confirmed diagnosis of MDS treated with HMAs were identified from the Michael E. Medical Center Cancer Registry. Patients were included if at least 2 G-banding metaphases studies were available and corresponded to: (1) karyotyping at disease diagnosis; and (2) at the time of azanucleoside failure. Overall Survival (OS) was analyzed for pts who have received HMAs with and without evidence of CE. Multivariate Cox regression analysis was performed to assess the impact of multiple independent variables on clinical outcome.
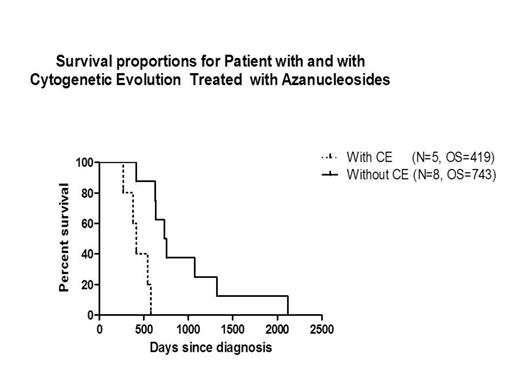
The incidence of CE in patients treated with HMAs was 38%. Median R-IPSS scores at diagnosis for pts with and without CE were 7.5 (5-very high; WHO subgroups: RCMD [2] and RAEB-2 [3]) vs. 4.75 (4-high and 4-Int; WHO subgroups: RCMD [3], RCUD [1] CMML [2]; MDS/MPN [2]) P=0.003. OS for pts with and without CE treated with HMAs was 419 days (d) vs 743 d, respectively P=0.001;CI= 0.23-0.89. In patients with CE, univariate analysis identified platelets and blast count at disease initiation (P=0.03, each) as prognostic variables impacting clinical outcome; however, by multivariate analysis a non-statistically significant trend was observed only for R-IPSS, P=0.21. (Table 1).
Impact of Independent Variables on Survival in Patients Receiving Azanucleosides and Experiencing CE.
| . | Univariate (P) . | Multivariate . |
|---|---|---|
| Platelets (K/uL) | 0.03 | 0.78 |
| Hemoglobin (g/dL) | 0.89 | 0.45 |
| ANC (K/uL) | 0.06 | 0.46 |
| Blast (%) | 0.03 | 0.42 |
| Age (years) | 0.36 | 0.57 |
| R-IPSS | 0.09 | 0.21 |
| . | Univariate (P) . | Multivariate . |
|---|---|---|
| Platelets (K/uL) | 0.03 | 0.78 |
| Hemoglobin (g/dL) | 0.89 | 0.45 |
| ANC (K/uL) | 0.06 | 0.46 |
| Blast (%) | 0.03 | 0.42 |
| Age (years) | 0.36 | 0.57 |
| R-IPSS | 0.09 | 0.21 |
In our retrospective analysis, acquisition of CE at the time of azanucleoside failure was associated with unfavorable outcome. Exploratory logistic regression analysis suggests that high-risk disease biology at disease initiation modulates incidence of CE in MDS pts treated with azanucleosides. Larger coalesced cohort of MDS pts experiencing CE could facilitate understanding of mechanisms associated with acquisition of genomic instability during azanucleoside failure and assist in identification of novel MDS targeted therapies that could ensure sustained response.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal