Abstract
Deregulation of apoptosis contributes largely to the pathogenesis of lymphoid neoplasms allowing the expansion of cell clones that develop resistance to drug-induced cell death. Therefore, subverting inherent resistance to apoptosis is a very challenging therapeutic issue and developing small molecules that can mimic the BH3 domain of the pro-apoptotic Bcl-2 family proteins appears to offer such potentials. Natural products, a rich source of compounds with BH3 mimetic activity, make a sound basis for such an approach. Quercetin, a natural polyphenol, has drawn much attention because it exerts anticancer cytotoxic effects, while sparing normal cells. Pro-apoptotic effects of quercetin have been shown in human lymphoma and leukemic cells and have been associated with Bcl-2 and Mcl-1 downregulation and Bax conformational activation. We undertook a multidisciplinary approach to elucidate the mode of action of quercetin, including cytotoxicity cell assays, biochemical approaches (pull down assays), NMR spectroscopy and docking calculations. We demonstrate that quercetin binds directly to the BH3 domain of the anti-apoptotic Bcl-2 and Bcl-xL proteins, exhibiting BH3-mimetic properties. Specifically, functional cytotoxicity assays of quercetin in Jurkat T-cell leukemic lines, Jurkat Bcl-2 and Jurkat Puro, indicated that quercetin binds to Bcl-2 protein. The direct binding of quercetin to Bcl-2 and to Bcl-xL was biochemically validated using pull down assays. NMR chemical shift perturbation experiments and docking calculations finally revealed that quercetin was bound to the pro-apoptotic BH3-binding site of the anti-apoptotic Bcl-2 family proteins (Figure 1). This property classifies quercetin as a natural flavonoid with BH3 mimetic activity, capable of driving leukemic cells to apoptosis. These results explain the cytotoxic effects of quercetin on Jurkat cells. These data can serve as a basis to consider studying BH3 mimetic natural products as adjuncts in the therapeutic approaches of lymphoid neoplasms in combination with chemotherapy, and can also provide a starting structure for developing novel potent analogues for the treatment of blood cancers. Close modal
Figure 1
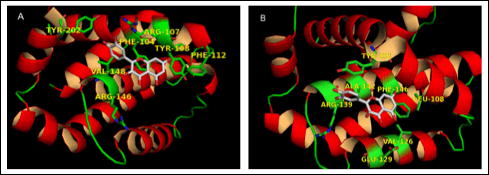
Modeling (3D) the complex of quercetin with Bcl-2 (A) and Bcl-xl (B). Quercetin occupies the deep hydrophobic cleft in both proteins making an extended interaction network. The relevant residues are normally occupied by the pro-apoptotic Bak/Bad BH3 binding site in the complex
Figure 1
Modeling (3D) the complex of quercetin with Bcl-2 (A) and Bcl-xl (B). Quercetin occupies the deep hydrophobic cleft in both proteins making an extended interaction network. The relevant residues are normally occupied by the pro-apoptotic Bak/Bad BH3 binding site in the complex
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal