Abstract
With the increased use of first generation novel therapeutic agents and extended life expectancy of multiple myeloma, patients’ Health-Related Quality of Life (HRQoL) is gaining considerable importance, including the relapsed/refractory multiple myeloma (RRMM) setting. So far, few studies have been conducted to appreciate the physical, psychological/cognitive, financial, social health impact of living with RRMM on longer term exposure to anticancer drugs.
The objective of this study was to evaluate the psychometric properties of the EORTC Quality-of-Life Core Questionnaire (QLQ-C30) and QLQ-Multiple Myeloma (QLQ-MY20) in RRMM patients.
A European, multicenter, observational study is being conducted in RRMM patients starting 2nd or 3rd line treatment. Patients are asked to complete the QLQ-C30 and QLQ-MY20 at baseline, month 3, and month 6 or discontinuation visit. Both generic and specific modules of EORTC questionnaires are widely used to assess HRQoL and symptoms in cancer patients. The QLQ-C30 includes 15 domains (Global Health Status/QOL, Physical Functioning, Role Functioning, Emotional Functioning, Cognitive Functioning, Social Functioning, Fatigue, Nausea and Vomiting, Pain, Dyspnea, Insomnia, Appetite Loss, Constipation, Diarrhea and Financial Difficulties); and the QLQ-MY20 includes four domains (Disease Symptoms, Side-Effects of Treatment, Body Image and Future Perspective). Construct validity was evaluated at baseline by confirming the structure using multitrait analysis and by assessing clinical validity against ECOG performance status. Internal consistency reliability was evaluated at baseline using Cronbach’s alpha.
As of June 2013, 206 patients have been enrolled in the study and included in this interim analysis. Mean age was 69 years, with 51% male, and with an average time since diagnosis of 3.4 years. A total of 90% of patients started 2nd line treatment and 10% started 3rd line treatment. Both EORTC questionnaires were well completed by patients, with 95% of patients responding to the QLQ-C30 and MY20 at baseline and 74% and 66% of patients, respectively, completing all QLQ-C30 and QLQ-MY20 items at baseline. Substantial percentages of patients reported the best possible level of HRQoL and symptoms at baseline, i.e. zero points for Appetite Loss (61%), Constipation (51%), Diarrhea (80%), Financial Difficulties (73%) and Nausea and Vomiting (73%), respectively 100 score points for Body Image (61%). The structure of multi-item QLQ-C30 and QLQ-MY20 domains was confirmed, as was the relevance of aggregation of items into domains (Table 1
Item-scale correlations and internal consistency reliability at baseline for multi-item domains
| EORTC . | Domain . | Number of items . | Item-scale correlations . | Internal consistency reliability (Cronbach’s alpha*) . |
|---|---|---|---|---|
| QLQ-C30 | Cognitive Functioning | 2 | 0.58-0.58 | 0.70 |
| Emotional Functioning | 4 | 0.66-0.75 | 0.85 | |
| Global Health Status/QoL | 2 | 0.88-0.88 | 0.93 | |
| Physical Functioning | 5 | 0.64-0.80 | 0.89 | |
| Role Functioning | 2 | 0.77-0.77 | 0.86 | |
| Social Functioning | 2 | 0.73-0.73 | 0.84 | |
| Fatigue | 3 | 0.75-0.79 | 0.87 | |
| Nausea and Vomiting | 2 | 0.54-0.54 | 0.63 | |
| Pain | 2 | 0.78-0.78 | 0.87 | |
| QLQ-MY20 | Future Perspective | 3 | 0.64-0.75 | 0.83 |
| Disease Symptoms | 6 | 0.47-0.79 | 0.81 | |
| Side-Effects of Treatment | 10 | 0.20-0.55 | 0.70 |
| EORTC . | Domain . | Number of items . | Item-scale correlations . | Internal consistency reliability (Cronbach’s alpha*) . |
|---|---|---|---|---|
| QLQ-C30 | Cognitive Functioning | 2 | 0.58-0.58 | 0.70 |
| Emotional Functioning | 4 | 0.66-0.75 | 0.85 | |
| Global Health Status/QoL | 2 | 0.88-0.88 | 0.93 | |
| Physical Functioning | 5 | 0.64-0.80 | 0.89 | |
| Role Functioning | 2 | 0.77-0.77 | 0.86 | |
| Social Functioning | 2 | 0.73-0.73 | 0.84 | |
| Fatigue | 3 | 0.75-0.79 | 0.87 | |
| Nausea and Vomiting | 2 | 0.54-0.54 | 0.63 | |
| Pain | 2 | 0.78-0.78 | 0.87 | |
| QLQ-MY20 | Future Perspective | 3 | 0.64-0.75 | 0.83 |
| Disease Symptoms | 6 | 0.47-0.79 | 0.81 | |
| Side-Effects of Treatment | 10 | 0.20-0.55 | 0.70 |
Recommended threshold for satisfactory internal consistency reliability: 0.70
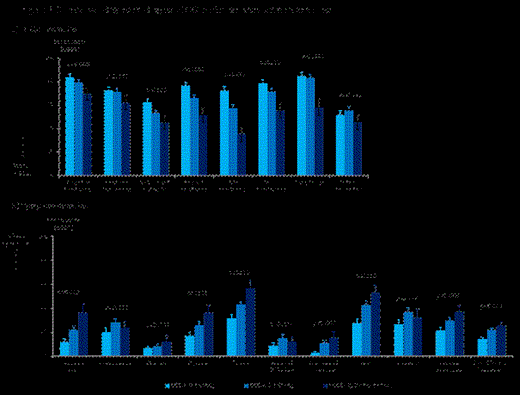
). Both QLQ-C30 and QLQ-MY20 scores were correlated to ECOG performance status (Figure 1).
This interim analysis confirmed in RRMM patients the satisfactory psychometric properties of the EORTC QLQ-C30 and QLQ-MY20 in terms of validity and reliability, already published in other cancer conditions. Psychometric properties of both questionnaires will be confirmed when repeating the analyses on the final dataset of this study.
Leleu:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Onyx: Consultancy, Honoraria; Leopharma: Consultancy, Honoraria; Millennium: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Petrucci:Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Welslau:Celgene: Membership on an entity’s Board of Directors or advisory committees; Janssen: Membership on an entity’s Board of Directors or advisory committees. Bottomley:Celgene: Consultancy, Research Funding. Bacon:Celgene International: Employment, Equity Ownership. Lewis:Celgene GmbH: Employment, Equity Ownership. Gilet:Celgene: Consultancy. Arnould:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal