Abstract
Tumor-specific markers and lifestyle factors have been shown to affect the outcomes of patients (pts) with FL. However, potential interactions and added prognostication connecting lifestyle factors and tumor markers have never been studied.
From a population-based prospective study of 123 FL pts (n=45 treated from 1983-1986 and n=68 from 1999-2002), we assessed the association between lifestyle factors (i.e. smoking, diet, and BMI) and tumor markers (i.e., CD68, CD7, FOXP3, CD10, and Ki67) and examined the individual and collective impact on 10-year overall survival (OS). Lifestyle habits were assessed through validated questionnaires and scoring of tissue microarrays were completed by expert pathologists (AC, DDW). The median age of all pts at diagnosis of FL was 61 years (34-92); 68% had stage III/IV disease; and the median follow-up of both groups of pts was more than 7.5 years.
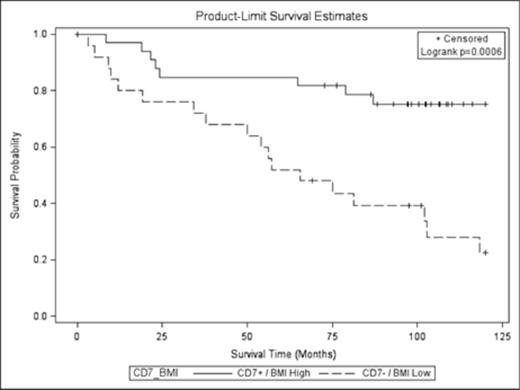
We identified several novel associated between lifestyle habits and tumor makers. Among all pts, high CD7 microenvironment expression was associated with a dietary pattern high in fruits, vegetables, and starch (P=0.046), as well as more specifically a higher intake of carotene-rich vegetables (P=0.005). Further, current smoking at diagnosis of FL was inversely related to levels of CD7 at diagnosis (i.e., smokers had lower CD7 levels, P=0.02). Overall fruit consumption was also associated with high CD10 expression (P=0.049). In addition, obesity was inversely associated with CD68 levels (i.e., high BMI associated with low CD68 levels, P=0.02). When examining lifestyle habits or tumor makers as singular prognostic factors, high BMI (above median) was associated with improved OS (HR 0.5 [95% CI 0.3-0.9], P=0.03) and high CD7+ expression was associated with improved OS (HR=0.50 [95% CI 0.3-0.8], P=0.005) as shown in Figures 1 and 2, respectively. When examining outcomes with lifestyle factors and tumor markers combined, there was a collective impact on outcomes, in particular with BMI. When CD7 expression and BMI were combined (Figure 3), the effect on OS was more prominent (i.e., CD7+ expression and high BMI, HR 0.3 [95% CI 0.1-0.6], P=0.0006). Additionally, results were controlled for time period with OS that included adjustment for age, sex, and education. The collective impact/association of CD7 and BMI remained significant (P=0.02), while new significant interactions emerged such as ‘current smokers’ with positive CD68 expression in microenvironment (OS HR 3.9 [95% CI 1.3-11.6] P=0.01). These associations and interactions transcended the time period of FL diagnosis.
Impact of BMI (high vs low, above/below median) on FL OS.
Impact of BMI (high vs low, above/below median) on FL OS.
Impact of CD7 expression (in FL tumor samples) on FL OS.
Collective impact of BMI and CD7 expression on FL OS.
We identified several novel lifestyle/tumor associations in FL including the association of CD7+ microenvironment with diets high in fruits and vegetables. Conversely, smokers presented with lower expression levels of CD7+ (i.e., T-cells) at FL diagnosis. In addition, we found that high expression of CD7 and high BMI were associated with significantly improved OS, while CD68 expression was associated with poorer OS. Furthermore, there were several lifestyle/tumor-related factors that had an additive effect on survival. Continued examination of the effect that lifestyle factors have on tumor cells and the microenvironment as well as their collective impact on survival is needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal