Abstract
Proteins within the complement system have complex effects on cellular immune responses. In previous studies, we found that active complement components, especially C5a, can dampen the development of antigen-specific immune responses following vaccination with a model antigen, in part by promoting generation of APC-induced T regulatory (Treg) cells. These studies also demonstrated that B lymphoma cell lines exposed to complement can induce Treg generation in vitro. The current study was designed to address whether depletion of C5a could enhance development of a cellular anti-lymphoma immune response in vivo.
Immunocompetent Balb/C mice were inoculated subcutaneously with syngeneic A20 B lymphoma cells mixed with either 10 μg of rat anti-mouse C5a monoclonal antibody (mAb) or 10 μg of isotype-matched Rat IgG2a control mAb. Tumor growth was followed. In select experiments, mice were sacrificed and analyzed for the percentage and activity of tumor-infiltrating T cells and A20-specific splenic T cell responses.
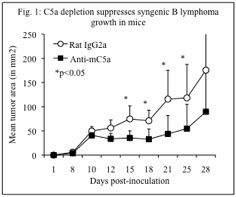
1. Tumor progression. Lymphoma grew more slowly in mice treated with anti-C5a mAb compared to mice treated with control mAb (p<0.05) {Fig. 1).
2. Intratumoral T cells. Tumors from mice treated with anti-C5a mAb had higher CD8+ T cell infiltration compared to mice treated with control mAb (p=0.002) (Fig. 2). Tumor-infiltrating CD8+ T cells showed a trend towards higher intracellular IFNg production in mice treated with anti-C5a mAb compared to control mAb (p=0.051).
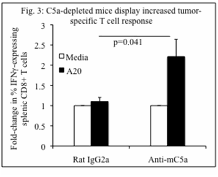
3. Splenic T cells. Splenic T cells from mice treated with anti-C5a mAb produced IFNg to a greater degree than did splenic T cells from control mice when splenocytes were cultured with irradiated A20 cells in vitro (p=0.041) (Fig. 3). There was a trend towards decreased numbers of splenic CD4+CD25highFoxp3+ Tregs in C5a-depleted mice compared to control mice.
Depletion of C5a at the site of tumor inoculation slows tumor growth and increases the number of tumor infiltrating CD8 T cells in a syngenic immunocompetent model of lymphoma. A trend towards enhanced production of IFNg in the tumor infiltrating T cells, increased numbers of tumor-specific splenic T cells, and reduced numbers of splenic Tregs, suggests intratumoral C5a depletion can enhance tumor-specific immune responses both within the tumor and systemically. Ongoing studies are exploring the molecular mechanisms involved in C5a-promoted tumor progression and the use of C5a depletion as a novel strategy to improve anti-tumor immunity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal