Abstract
CD57+ NK cells are a subset of CD56lo/CD16+ NK cells which have been alternatively described as terminally differentiated or memory NK cells; however their physiological role remains unclear. In this study, we explored this unique NK subset and define their role in the context of MM.
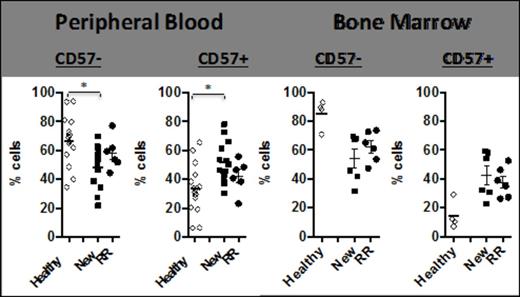
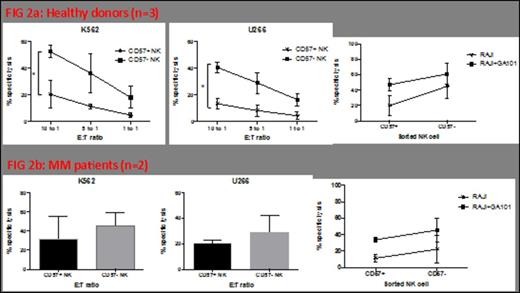
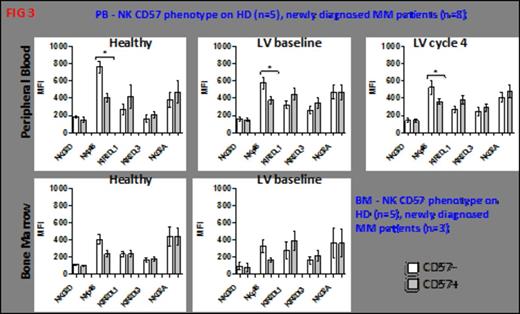
CD57+ NK cell frequency was initially assessed by FACS. Thus, newly diagnosed untreated (NEW) Multiple Myeloma (MM) patients had a significantly increased frequency of CD57+ NK cells in the PB (p=0.0029) and BM (p=0.0095) compared with age matched normal donor controls. Refractory relapsed (RR) MM patients also had significantly increased CD57+ cells in the BM (p=0.008) (Figure 1). There was no change in CD57+ NK cell numbers (in either patient cohort) following three cycles of lenalidomide+dexamethasone (len+dex) therapy. Normal donor CD57+ NK cells had significantly less natural cytotoxicity function (compared with CD57- NK cells) against K562 (p=0.04) or U266 (p=0.0265) (Figure 2A). The same trend was observed in MM patient CD57+ NK cells; however this did not reach statistical significance (Figure 2B). In contrast, the ADCC activity for CD57+ and CD57- NK cells was the same whether the effector cells were from normal donors (Figure 2A) or MM patients (Figure 2B). To explain the reason for this difference in natural cytotoxicity function between the two NK cell subsets, the balance of NK activating (KAR) and inhibitory receptors (KIR) was examined (Figure 3). CD57+NK cells expressed significantly lower levels of the NK activating receptor Nkp46 than CD57-NK cells, in the PB of normal donors (p=0.001), NEW MM patients at presentation (p=0.008) or NEW MM patients after three cycles of len-dex therapy (p=0.0237) (Figure 3). In contrast, both CD57+ and CD57- NK cell subsets in RR MM patients had the same levels of Nkp46, this did not change with len-dex therapy. To further explore reasons for the CD57+ NK cell functional state, expression of NK effector molecules (perforin and granzyme) and NK effector transcription factors (TF) T-bet and Eomesdermin (Eomes) was assessed. Surprisingly, PB CD57+ NK cells had significantly higher perforin expression than CD57- NK cells in the PB of normal donors (p=0.0089) and NEW (p=0.0815) MM patients, and the BM of NEW MM patients (p=0.0487). Similarly, granzyme B was significantly elevated in CD57+ compared to CD57- NK cells in normal donors (p=0.013), NEW MM patients in the PB (p=0.0024) and BM (p=0.0007). There was no statistical difference in the expression of Eomes or T-bet in either NK subset, whether the cells were from normal donors or MM patients. Nonetheless, a trend in higher Eomes expression was observed in CD57+ NK cells from MM patients compared to age matched controls. Lastly, CD57+ (compared to CD57-) NK cells have a significantly lower proliferation rate in a NK homeostatic proliferation assay.
Multiple myeloma patients have a higher frequency of CD57+ NK cells in the peripheral blood and bone marrow.
Multiple myeloma patients have a higher frequency of CD57+ NK cells in the peripheral blood and bone marrow.
CD57+ NK cells elicit a different cytotoxicity profile to the CD57- NK subset.
CD57+ NK cells express lower levels of the critical cytotoxic receptor NKp46.
Studies to date indicate CD57+ NK cells are terminally differentiated NK cells; however to date their role remains undefined. We observed an increased frequency of CD57+ NK in the PB and BM of newly diagnosed untreated MM patients. These CD57+ NK cells have the same functional and phenotypic features in normal donors and MM patients, whether at diagnosis or in RR patients. The strikingly reduced natural cytotoxicity in CD57+ NK cells, coupled with reduced Nkp46 expression, indicates these cells are not important for surveillance of viral infected or tumour cells. Rather CD57+ NK express elevated effector proteins and the effector TF Eomes and are ‘primed’ for killing of target cells via ADCC. Unlike CD57- NK cells, CD57+ NK cells do not undergo homeostatic proliferation; whilst this is consistent with terminally differentiated cells, it is not clear how these cells persist in the periphery of MM patients. Nonetheless, CD57+ NK cells are elevated in MM patients at presentation, whilst this likely contributes to the overall decreased natural cytotoxicity observed in MM patients, it also provides an opportunity to activate NK cells via CD16 (FcγRIII) to induce ADCC against MM.
Harrison:Celgene Corp: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding; Virolytics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal