Abstract
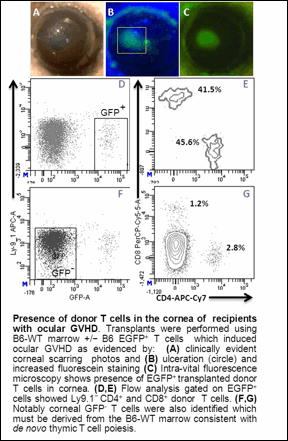
Allogeneic hematopoietic stem cell transplantation (HSCT) has become the standard of care for the treatment of several life-threatening hematologic malignancies as well as certain immunodeficiency disorders. Unfortunately, as the survival rate of patients with these diseases is improved, the quality of life is negatively impacted by the development of Graft vs. Host Disease (GVHD). GVHD is a complex, multi-organ disorder arising from an immunological attack by donor allo-reactive T cells that results in damage to vital organs including the liver, skin, hematopoietic compartment and the ocular surface of the eye. Ocular GVHD occurs in >60% of these patients and is characterized by dry eye, conjunctiva damage, punctate keratopathy, corneal ulceration and perforation. Despite the high frequency of eye involvement in patients undergoing GVHD, little is known regarding the underlying immune mechanisms responsible for ocular GVHD, limiting the ophthalmic care of these patients to palliative therapies and global anti-inflammatory drugs. In this study, we examined the ocular and immunological changes occurring in recipients of MHC-matched, minor antigen mis-matched donor HSCT. C3H.SW (H-2b, Ly9.1+) mice transplanted with EGFP+ B6 (H-2b, Ly9.1-) T cell depleted bone marrow cells (TCD-BM) supplemented with T cells: a) underwent weight loss and began exhibiting clinical signs of GVHD ∼3wks post-HSCT, b) contained damaged thymuses, c) expressed an inverted CD4/CD8 ratio in the peripheral lymphoid compartments, d) contained activated effector cells and e) low CD19 levels. Importantly, these mice also developed ocular surface disease evidenced by progression of ocular surface damage characterized by increased corneal fluorescein staining and ulceration by week 6 (Figure). Furthermore, histological analyses demonstrated that only mice that developed systemic GVHD exhibited corneal thickening and epithelial irregularity. Ocular pathology was also associated with conjunctiva involvement indicated by significant goblet cell destruction as well as dense inflammatory cell infiltrates identified by intra-vital fluorescent microscopy (Figure). IHC and flow analyses demonstrated donor EGFP+ Ly9.1- CD4+ and CD8+ T cells. Notably, significant levels of Ly9.1- EGFP-CD11b+ macrophages had also infiltrated the ocular surface. In contrast to the systemic CD8>CD4 GVHD associated phenotype, the T cell infiltrate in the ocular compartment was reversed; i.e. CD8<CD4. We detected IFNγ and TNFα mRNA from corneal tissue, which is consistent with Th1 effector allo-reactive cells and M1 inflammatory macrophages involvement in ocular GVHD. In total, the present findings have identified alterations and pathology in the eye and adnexa reflective of ocular GVHD and unequivocally demonstrate the presence of donor T cells in the ocular surface. We hypothesize that T cell–macrophage interactions underlie the pathology detected in this pre-clinical model and studies are underway to develop local therapeutic modalities targeting these infiltrative populations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal