Abstract
The recipients of solid organ transplants are infrequent candidates for allogeneic hematopoietic stem cell transplantation (alloHSCT). However, such situations occur more frequently with increasing number of solid organ transplantation (SOT) survivors who may suffer from hematopoietic disorders. AlloHSCT may cause immune reactions towards transplanted organ leading to its failure. Since the reported clinical experiences have been anecdotal, outcomes of alloHSCT after SOT are not well known. In order to document the feasibility of alloHSCT after SOT, we decided to perform a retrospective analysis of patients reported to the EBMT registry.
The only inclusion criterion was alloHSCT performed after SOT. An invitation to participate was sent to all EBMT transplant centers (N=565). In 18/101 centers which responded to the survey, we identified 28 patients who fulfilled the criterion. This group included 12 patients with kidney (1 case with subsequent pancreatic transplantation), 13 with liver and 3 with heart transplant. The reason for heart transplantation was insufficiency due to multiple myocardial infarctions, anthracyclin-associated myocarditis or dilated cardiomyopathy. Liver transplantations were performed for hepatocellular cancer (N=4), cirrhosis without cancer (N=4), sclerosing cholangitis (N=1), acute liver failure after chemotherapy (N=1) or idiopathic liver failure (N=3). The cause of kidney transplantation was pyelonephritis (N=3), glomerulonephritis (N=2), polycystic disease (N=1), tubulointerstitial nephropathy (N=1), diabetic nephropathy (N=1) or idiopathic kidney failure (N=4). The indications for alloSCT were: acute leukemia (N=10), chronic leukemia (N=4), lymphoma (N=3), myelodysplastic/myeloproliferative disease (N=4), bone marrow failure (N=4) and inherited disorders (N=2). The median time from SOT to hematologic diagnosis was 23 months (range, -206 to 294), in 8 patients the diagnosis was known before SOT. Twenty eight SOT recipients underwent 31 alloHSCT (3 patients underwent double alloHSCT after SOT), while in 2/28 alloHSCT was preceded by autoSCT. The median year of alloSCT was 2006 (range, 1990-2012) and the median time between SOT and alloHSCT was 35 months (range, 1-315). The conditioning was standard (N=16) or reduced (N=14) and included TBI in 12 cases, but the regimens were heterogeneous. The HSCT was performed with either peripheral blood stem cells (N=20), bone marrow (N=9) or both (N=1) obtained from HLA-identical sibling (N=12), matched (N=11) and mismatched (N=3) unrelated or haploidentical (N=5) donors. T cell depletion was performed in 20/29 cases (in vivo N=15, ex vivo N=2, in vivo and ex vivo N=3). The most common GvHD /SOT rejection prophylaxis was cyclosporine A and methotrexate (N=12) followed by cyclosporine A and mycophenolate (N=5), tacrolimus and mycophenolate (N=5) and tacrolimus with methotrexate (N=1), but frequently included also other immunosuppressive drugs, especially steroids.
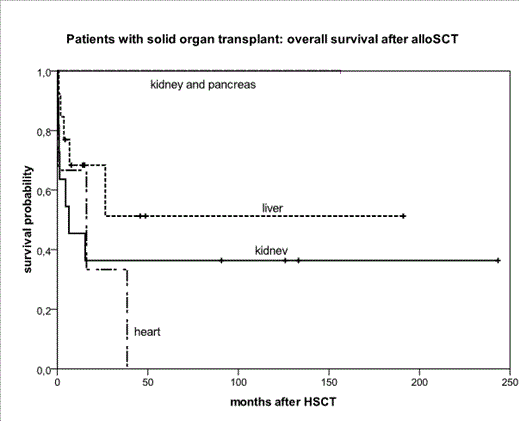
Hematopoietic engraftment was achieved after 25/30 transplantations including 2 cases where the graft was lost. In evaluable patients, time to neutrophil recovery (>0.5 G/L) was 13.5 days (9-37, N=22), in 3 patients the platelet count never fell below 20 G/L and the rest recovered after median of 17 days (range, 10-103). Acute GvHD was observed in 17/31 cases including 10 with grade II-IV disease. Terminal solid organ transplant failure was observed in 10 of 30 alloHSCTs (1/3 heart, 3/13 liver and 6/11 kidney transplants) after median of 6 weeks (range, 0-569) after HSCT (8/10 in early post-HSCT period). Six of 10 failure cases have been described as SOT rejections (4 kidneys, 1 liver, 1 heart). After median of 14 months from alloHSCT (range, 0,3-244) 13/28 patients were alive (46%, 8/13 patients after liver, 5/12 after kidney and none after heart transplantation). The estimated probability of survival after HSCT was 75% at 3 months, 60% at 12, 45% at 36, and 40% at 60 months. In one third of patients (5/15), death was directly related to transplanted solid organ failure.
In summary, a significant proportion of patients enjoy long term survival without graft loss, especially after liver transplantation. Therefore our results suggest that alloHSCT is a feasible treatment option for patients after SOT. However, the risk of transplanted solid organ failure is high, including the possibility of solid organ rejection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal