Abstract
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) from haploidentical donors (HID) as alternative donor is currently increasing for patients with hematological diseases. In this setting, post Allo-HSCT high dose Cyclophosphamide (Cy) allows the use of T-repleted graft from HID by decreasing early alloreactive T-cell, leading to low incidences of graft-versus-host disease (GVHD) and non-relapse mortality. However, the efficacy of this procedure is still a matter of debate. The aims of this study were to evaluate the efficacy of HID Allo-HSCT in a comparison with our historical control cohort of patients transplanted from matched related or unrelated donors.
We report here the experience of HID Allo-HSCT prepared by reduced intensity conditioning (RIC) regimen and post infusion Cy (HID group; n=60) and compared outcome to that of historical control cohort of patients with hematological diseases (n=220) who underwent RIC Allo-HSCT from matched related donors (MRD group; n=110) or unrelated donors (UD group; n=110). The control cohort received RIC regimen based on Fludarabine (150 mg/m2 total dose over 5 days), intravenous Busulfan (6.4 mg/kg total dose over 2 days) and antithymocyte globulin (ATG; Thymoglobulin; 5 mg/kg total dose over 2 days). To better compare the results between these two heterogeneous cohorts of patients including different hematological diseases, we used the disease risk index (DRI) score recently published (Armand at al., Blood, 2012).
Patient characteristics are illustrated in the Table 1. Although HID and MRD/UD groups were significantly different regarding age and graft source, they presented with similar disease risk profile when classified according to the DRI and comorbidities according to the HCT-CI.
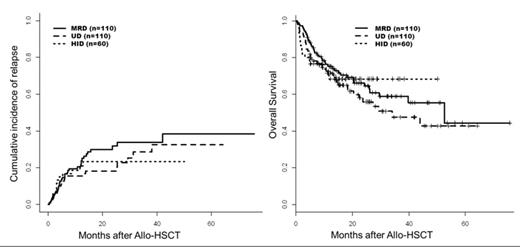
In the HID group, day-100 acute GVHD, overall incidence of chronic GVHD and 2-year NRM were 21%, 9% and 15% respectively. Regarding the disease control, with a median follow up of 17 months (range: 5-50), the 2-year cumulative incidence of relapse (CIR) was 23%, which is similar to the MRD and UD control groups (2-year CIR: MRD 31%; UD: 18%). The 2-year PFS and OS were 62% and 68% respectively in the HID group, 52% and 66% respectively in the MRD group and 55% and 56% respectively in the UD group.
These results suggest that RIC Allo-HSCT from HID allows promising anti-tumor effect that is comparable to our previous experience of Allo-HSCT from MRD or UD. We also confirm the low toxicity profile after HID Allo-HSCT. Taken together, these observations suggest that HID is a valuable alternative for patients with hematological diseases but no available MRD or UD.
Cumulative incidence of relapse and overall survival after HID RIC Allo-HSCT and in the historical control MRD and UD cohorts
Cumulative incidence of relapse and overall survival after HID RIC Allo-HSCT and in the historical control MRD and UD cohorts
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal