Abstract
Patients with sickle cell disease (SCD) are known to have altered immune systems. In the past, immunology studies in SCD patients have focused largely on splenic dysfunction and the increased risk of infection with encapsulated organisms. Increasingly, however, it is now being recognized that SCD patients also have evidence of abnormal immune activation with baseline chronic inflammation as well as high rates of red blood cell (RBC) alloimmunization and hematopoietic stem cell transplant (HSCT) graft rejection. We have previously demonstrated an increased risk of bone marrow transplant rejection with non-myeloablative conditioning in a SCD mouse model, with both increased T cells and natural killer (NK) cells implicated in this rejection. We have also previously shown that at baseline children with SCD have altered immune cell subsets. We now have expanded on this investigation of quantitative immune deviation in pediatric SCD by exploring how chronic transfusion (CT) or hydroxyurea (HU) therapy affects immunophenotype in a larger cohort of patients.
One hundred and eleven children (68 patients on CT only, 26 patients on HU only, 17 patients on neither CT nor HU) with SCD (SS or Sβ0) and 29 healthy, age-matched African American controls were recruited for this study. Exclusion criteria included recent illness or another disease associated with known abnormalities of the immune system. Flow cytometry was performed on samples from all participants to quantify the following immune cell populations: total white blood cells (WBC), total monocytes, total lymphocytes, T cells (total, CD4+, CD8+, naïve, central memory, effector memory, effector memory RA, and CD4+ T regulatory), B cells (total, naïve, and memory) and NK cells.
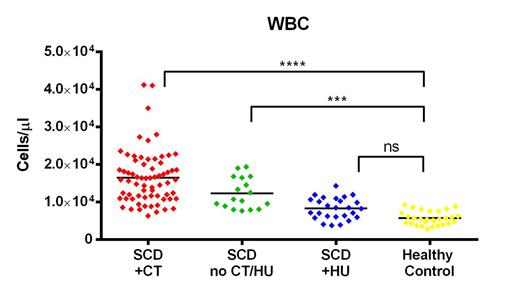
SCD patients on CT and patients not receiving CT or HU (no CT/HU) had significantly increased total WBCs, granulocytes, monocytes, lymphocytes, T cells and B cells compared to healthy controls. Conversely, while SCD patients on HU also had mean counts that were elevated, they were not significantly greater than controls for any of these cell populations (Figure 1). Similarly, CD4+ T cells were significantly increased only in the CT and no CT/HU patients compared to controls (Figure 2). CD8+ T cells, however, were increased only in the CT group. CD4+ T cell subset analysis showed the most striking elevations in the CD4+ central memory and CD4+ effector memory (TEM) populations for both CT and no CT/HU patients compared to controls (Figure 3). CD4+ T regulatory cells were also increased in both of these groups. B cell analysis showed increased numbers of naïve but not memory B-cells in CT and no CT/HU patients. In distinct contrast to most populations that were elevated in CT patients, cytotoxic NK cells were uniquely increased only in no CT/HU patients.
Children with SCD have quantitative differences in many different immune cell populations compared to age and race matched healthy controls. HU treatment appears to bring the absolute number of these cell subsets closer to those of healthy controls, while CT does not. It is unclear at this point to what extent CT therapy itself or baseline patient characteristics leading to the need for CT contribute to these findings. Future work is investigating whether certain cell subset abnormalities are associated with particular therapeutic complications such as RBC alloimmunization or HSCT graft rejection. Better understanding the immunology of SCD may allow for targeted therapies to improve SCD transfusion support and HSCT outcomes.
Off Label Use: Abstract includes information related to the use of hydroxyurea in children with sickle cell disease.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal