Abstract
EPAG, an oral thrombopoietin receptor agonist approved for cITP, increased platelets (plts) and reduced bleeding in 6 wk and 6 m placebo-controlled trials in previously treated cITP patients. EXTEND is an ongoing, open-label extension study, begun in Jun 2006, to assess the safety and efficacy of long-term treatment with EPAG in cITP patients who completed a previous EPAG study. Long-term safety and efficacy data up to Feb 2013 are presented.
EPAG was started at 50 mg and titrated to 25-75 mg/d or less often, based on plt counts. Patients who received ≥2 y of EPAG and transitioned off due to commercial availability of EPAG were considered to have completed EXTEND, whether or not they continued treatment with commercial EPAG.
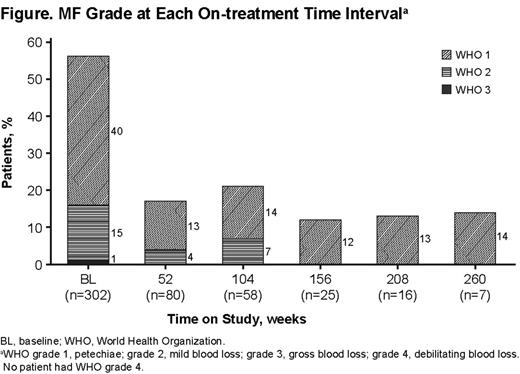
Of 302 patients enrolled, 43% (129) completed, 48% (146) withdrew, and 9% (27) remain on study. The most common reasons for withdrawal were adverse events (AEs; 15%), patient decision (13%), lack of efficacy (11%), and stable plts ≥6 m following interruption of EPAG (3%). The Table shows baseline patient characteristics and treatment duration. Doses of 75, 25, and 25 mg QOD were required by 62%, 51%, and 20% of patients, respectively, at some time during the study; 5% remained on 50 mg throughout the study (overall median duration of exposure, 122 wk; average dose, 50.8 mg/d). Overall, 85% (258/302) of patients achieved plts ≥50,000/µL in the absence of rescue therapy, and 61% achieved plts ≥50,000/µL for ≥50% of on-treatment assessments. Median plts increased to ≥50,000/µL by Wk 2, remaining consistently ≥50,000/µL throughout the treatment period. Nine of 10 patients who withdrew due to stable plts for ≥6 m following interruption of EPAG maintained plts ≥100,000/µL for ≥6 m without any ITP therapy. Incidence of bleeding symptoms (WHO grades 1-4) decreased from baseline to 1 y and thereafter (Figure ). Of 101 patients receiving concomitant ITP treatment at baseline, 40 had a sustained reduction or permanently stopped ≥1 concomitant ITP treatment without ever receiving rescue therapy. The most frequently discontinued/reduced ITP medications were corticosteroids (35/40; 88%) and danazol and azathioprine (4/40 each; 10%). In 92% (277) of patients, AEs occurred. Serious AEs (SAEs) occurred in 31% (94) of patients, and 22 patients had 33 SAEs considered possibly drug related. Drug-related SAEs occurring in ≥2 patients were cataracts (7), alanine aminotransferase (ALT) (4) or aspartate aminotransferase (2) increased, deep vein thrombosis (DVT; 4), bilirubin increased (3), myocardial infarction (MI; 2), and pulmonary embolism (PE; 2). AEs leading to withdrawal occurred in 44 patients (15%), 29 (10%) of whom experienced SAEs. The most frequent AEs leading to withdrawal were increased ALT (7), increased bilirubin (5), cataracts (4), and DVT (4). In 19 patients (6%), 26 thromboembolic events (TEEs) were reported (incidence rate, 2.53/100 patient y; 95% CI, 1.52-3.95). Observed TEEs were DVT (11), central nervous system ischemic events (7), MI (5), and PE (3). No association with elevated plt counts was observed, as only 3/19 patients experienced the TEE at or shortly after achieving their maximum plt count. Hepatobiliary laboratory abnormalities (HBLAs) were reported in 37 patients (12%), and 8 were withdrawn because of HBLAs. No HBLAs were associated with signs of liver impairment; most resolved on treatment or after discontinuation. An independent central pathology review of bone marrow (BM) biopsies stained for reticulin from 115 patients treated with EPAG for ≤5.5 y found no clinically relevant increase in reticulin deposition. Two patients (2%) had maximum reticulin marrow fibrosis (MF) grade of ≥2 after >24 m on treatment; neither experienced any AE or hematologic parameter abnormality potentially related to impaired BM function.
Sustained plt increases and reduced bleeding symptoms were observed in EPAG-treated cITP patients throughout the study. Sustained increases in plt counts were maintained in a few patients after discontinuing EPAG. Concomitant ITP medications were reduced without requiring rescue medications. Eltrombopag was well tolerated with exposures ≤6.5 y. Rates of TEEs and HBLAs did not increase with longer treatment duration, and BM biopsies showed no clinically significant increase in MF grade. No new safety signals were observed. Long-term safety and efficacy continue to be assessed in this ongoing study.
Bussel:Symphogen: Membership on an entity’s Board of Directors or advisory committees; Genzyme: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; IgG of America: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Eisai Inc.: Research Funding; Cangene: Research Funding; Amgen: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Wong:GlaxoSmithKline: Consultancy, Honoraria, Research Funding, Speakers Bureau. Burgess:GlaxoSmithKline: Employment, Equity Ownership. Bakshi:GlaxoSmithKline: Employment, Equity Ownership. Chan:GlaxoSmithKline: Employment. Bailey:GlaxoSmithKline: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal