Abstract
Juvenile Myelomonocytic Leukemia (JMML) is a devastating childhood cancer which is rapidly fatal with infiltration of myeloid cells into multiple organs (Hess, Zutter, Castleberry, & Emanuel, 1996). Based on the observation that uniparental disomy was found in the chromosomal region 11q in JMML patient samples, about 15% of patient samples were found to contain a mutation in c-Cbl (Loh et al., 2009). Moreover, mutant Cbl was also found to be a tumor suppressor gene where a germline mutation results in the predisposition for developing JMML (Niemeyer et al., 2010). The c-Cbl gene encodes a multifunctional adaptor protein which contains an N-terminal tyrosine-kinase binding (TKB) domain, a RING finger motif which contain E3 ligase activity, and a C-terminal ubiquitin-associated domain. Previous mutations in myeloid malignancies have been described where mutations occur in the RING finger domain or the linker domain (Caligiuri et al., 2007; Dunbar et al., 2008). Interestingly, a hotspot for mutations at residue 371 exists in JMML patients where 1/3 of the detected mutations are a tyrosine to histidine substitution, Y371H (Loh et al., 2009). This residue belongs in the linker region of the CBL protein, and it was previously observed that Y371 mediates the binding of c-Cbl to the p85 subunit of PI3 kinase (Blaydes et al., 2001). In vitro, CblY371H mutation does indeed destroy its ligase function resulting in prolonged signaling through the Ras pathway only when the endogenous c-Cbl gene was silenced (Niemeyer et al., 2010). However, how mutant Cbl gives rise to JMML and how it acts in concert with other genes in the pathogenesis of JMML requires further study.
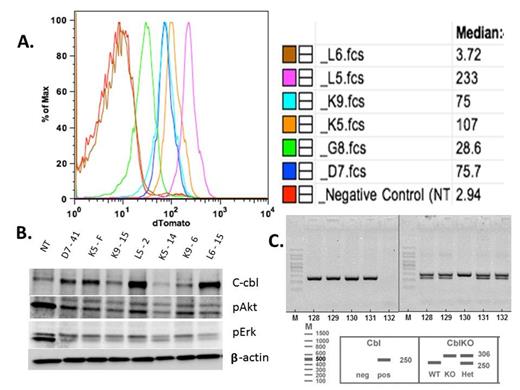
Generation and Initial Characterization of CblY371H Transgenic Mice A.Flow cytometry of splenocytes from the various transgenic lines. Panel A demonstrating tdTomato fluorescence from each of the transgenic lines generated. Note that there are expression differences among the 6 lines with line L5 expressing the highest amount of fluorescence and line L6 with no fluorescence. B.Western Blot of spleen extracts from transgenic mice Panel B confirms human Cbl protein expression from transgenic mice. L5 and L6 lines express the most protein followed by K5 and D7 as predicted based on Tdtomato expression in panel A except L6 C.Genotyping of mice for cbl ko and cbl transgene As seen in the figure, Cbl PCR and Cbl KO PCR is done separately with #130 showing that it carries the transgene for Cbl as well as being homozygous for Cbl KO allele.
Generation and Initial Characterization of CblY371H Transgenic Mice A.Flow cytometry of splenocytes from the various transgenic lines. Panel A demonstrating tdTomato fluorescence from each of the transgenic lines generated. Note that there are expression differences among the 6 lines with line L5 expressing the highest amount of fluorescence and line L6 with no fluorescence. B.Western Blot of spleen extracts from transgenic mice Panel B confirms human Cbl protein expression from transgenic mice. L5 and L6 lines express the most protein followed by K5 and D7 as predicted based on Tdtomato expression in panel A except L6 C.Genotyping of mice for cbl ko and cbl transgene As seen in the figure, Cbl PCR and Cbl KO PCR is done separately with #130 showing that it carries the transgene for Cbl as well as being homozygous for Cbl KO allele.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal