Abstract
Bone marrow stromal cells (BMSC) provide a suitable microenvironment for multiple myeloma (MM) cell survival, resistance and related osteolytic bone disease. Wnt signaling enhances osteoblast proliferation and mineralization, whereas it blocks osteoblast apoptosis and osteoclast differentiation by increasing the OPG/RANKL ratio. The Wnt inhibitor Dickkopf 1 (Dkk1) negatively regulates canonical Wnt signaling by binding to and antagonizing the Wnt co-receptors Lrp5/6 (He et al., 2004). Serum levels of DKK1 have been reported to be elevated in patients with MM, while osteoprotegrin levels are decreased (Tian et al 2003). Crosstalk between Wnt/β-catenin and other signaling pathways within the bone marrow microenvironment that regulate the osteogenic differentiation of mesenchymal stem cells (MSCs) has been reported previously (Cawthorne et al. 2012 and Krishnan et al. 2006). However the question of whether the pro-oncogenic effect of Wnt-β-catenin signal from the stroma antagonizes the elevated serum DKK1 and thus actually enhance the osteolytic actvities in MM relapse remains to be answered.
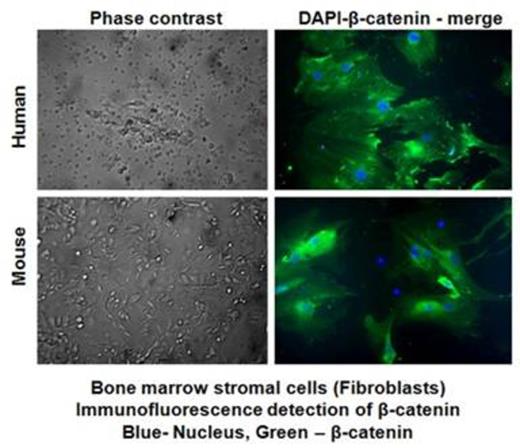
Bone marrow-derived stromal fibroblasts from myeloma patients were maintained in culture for 21 days. Adherent stromal fibroblast (confirmed with staining for Vimentin) and non-adherent cells were examined for DKK1 by ELISA and for Wnt β-catenin by performing (a) immunofuoresence detection, (b) luciferase reporter assays and (c) Western blot analysis.
Adherent stromal fibroblasts showed β-catenin expression after 21 days in culture (Fig 1), the signal for β-catenin expression was 68% compared to that in non-adherent BM cells (27%). Consistently, luciferase reporter assays of adherent stromal fibroblasts transfected with Wnt/β-cat responsive reporter plasmids (STF16) showed >4 fold increase in the reporter activity compared to that in the MM cells. As expected, the level of soluble DKK1 was significantly lower in patients with early MM (ranges from 5167 ± 379 pg/ml) vs. advanced stage MM (53967±1323 pg/ml) among patients who are relapsed (n=16), and the difference in the DKK1 levels between these two groups of samples (early vs. advanced stage MM, n=8 each) are statistically significant (p<0.05). The increase in serum DKK1 is also directly correlated with increase in the serum VEGF and vascular tube formation suggesting the stromal influence of Wnt/β-catenin in mediating angiogenesis in MM relapse patients. Transfection of stromal fibroblasts with siRNA for NF-κBp65 down regulated both β-catenin and DKK1 at the mRNA and protein levels, suggesting a close interaction between Wnt/β catenin and inflammatory signaling within the BM stromal microenvironment.To further determine the effect of potential drugs on the regulation of β-catenin and DKK1, we measured soluble DKK1 activity in the cell culture medium of growing BMSC cells stimulated with human M-CSF and RANKL to generate osteoclasts and osteoblasts, followed by treatment with different concentrations of Bortezomib, or Dipyridamole (an agent which increases exracellular adenosine levels) at a concentration of 2.5, 5.0 and 10ug/ml respectively) for 24h. As previously reported, dipyridamole increased obsteoblast differentiation, surprisingly, diminished sDKK1 levels, as well as bortezomib (11667±1528 pg/ml) at 10 ug/ml.
Our preliminary findings suggest that Wnt/β-catenin and sDKK1are co-produced in BM stromal cells and are both diminished by drugs that modulate both Wnt and RANKL pathways. Another interesting message from this study is that the stromal derived β-catenin may influence bone metastasis of myeloma, as seen in other cancer types, via transcriptionally regulating DKK1 within the BM niche. Since the bone marrow stromal cells from MM relapse patients still express an elevated level of β-catenin, in spite of the higher serum DKK1 level in MM relapse patients, we believe that stromal contribution of β-catenin is critical in patients with multiple myeloma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal