Abstract
The administration of ZA in patients with symptomatic myeloma is associated with reduction of SREs and possible improvement of survival; its use however, is associated with increased risk of osteonecrosis of the jaw (ONJ). Longer exposure and more infusions of ZA have been associated with higher incidence of ONJ. It has been suggested that longer intervals between ZA infusions may reduce the risk of ONJ; however, this has not been proven. Since 2008, we have adopted a strategy in which ZA is discontinued in patients who remain in remission for more than 2 years, while ZA is administered every 8-12 weeks, after the first year, in patients who have achieved vgPR or CR. The aim of our analysis was to assess the incidence of ONJ in a prospectively studied population treated with ZA and to asses potential risk factors related to dosing and schedule of ZA.
Since 2003, all patients in the Department of Clinical Therapeutics (Athens, Greece) undergo dental evaluation before ZA initiation and are instructed to avoid procedures that predispose to ONJ. Only patients who received ZA and survived at least 6 months post their first ZA infusion were included in the analysis. Relative dose frequency of ZA (RDF) was calculated as the average number of weeks between infusions of ZA (weeks between first and last infusion divided by the number of infusions). Time of exposure to ZA was calculated from the date of first infusion until the date of last infusion. Death due to myeloma and development of ONJ were treated as competing events.
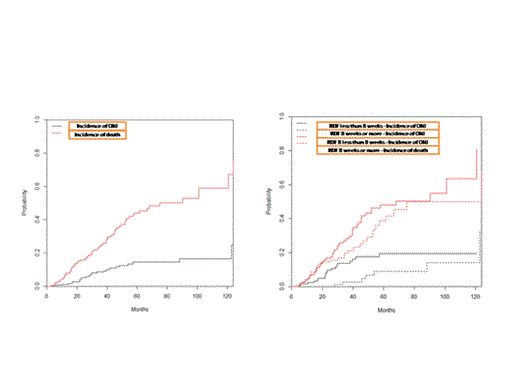
Between January 2000 and January 2013, 266 patients with symptomatic myeloma fulfilled the above criteria and were included in the analysis. Median age was 68 years (range 36-87 years) and 47% were males. Median follow up was 36 months and 35% of the patients have died. The median number of ZA infusions was 16 (range 1-107) and the median time of exposure to ZA was 29 months, corresponding to 9073 person-months of exposure. Median RDF was one infusion per 7.9 weeks (interquartile range (IQR) 5.8-11.4 weeks). ONJ developed in 26 (10%) patients. Median time from first ZA infusion to development of ONJ was 28 months (range 6-122 months). Accounting for death as a competing event, 1-year risk of ONJ was 1.2% (95% CI 0.3-3%), 2-year risk was 5% (95% CI 2.5-8.5%), 3-year risk was 8.5% (95% CI 5-13%), 4-year risk was 11.5% (95% CI 7.3-17%) and 5-year risk was 14% (95% CI 9-19%) (Figure). The respective survival rates were 93%, 84%, 76%, 65% and 56% at 1, 2, 3, 4 & 5 years. The median time of exposure to ZA was 28 months (IQR 22-46 months) for patients who developed ONJ vs. 24 months (IQR 11-42 months) for those who did not (p=0.2). However, the median number of ZA infusions was 23 for those who developed vs. 14 for those who did not develop ONJ (p=0.004). Increasing number of ZA infusions was associated with higher risk of ONJ: 2.3% of patients who received <10 infusions developed ONJ vs. 12% of patients who received 10-19 infusions vs. 15% of patients who received 20 or more ZA infusions (p=0.012). The incidence rate of ONJ for patients who started ZA before 2008 was 0.31 per 100 person-months vs. 0.26 per 100 person-months for those who started after 2008 (p=0.4). The incidence of ONJ at 3-years was 13.6% (95% CI 8-20%) for patients with an RDF <8 weeks vs. 2.6% (95% CI 0.5-8%) for patients with RDF of ≥8 weeks (p=0.018). However, after adjusting for RDF of ZA infusions, only the number of ZA infusions remained significant (p=0.03). The multivariate analysis showed that both the number and the frequency of ZA infusions were associated with a shorter time to ONJ development. More specifically, average frequency of infusion <8 weeks was associated with a 15-fold (95% CI 4-55, p<0.001) increase in the risk of ONJ, while for every infusion of ZA the risk of ONJ increased by 9% (95% CI 4-14%, p<0.001).
In conclusion, our data, in a large population of consecutive unselected patients with symptomatic myeloma who received ZA, indicate that ONJ remains a frequent complication in patients who receive ZA for prolonged periods and the risk increases with the number of ZA infusions. More frequent infusions of ZA are associated with earlier development of ONJ, but the risk of ONJ is associated mainly with the cumulative dose of ZA. Prospective clinical trials should examine if less frequent administration of ZA can reduce the risk of ONJ without compromising its antiresorptive effect.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal