Abstract
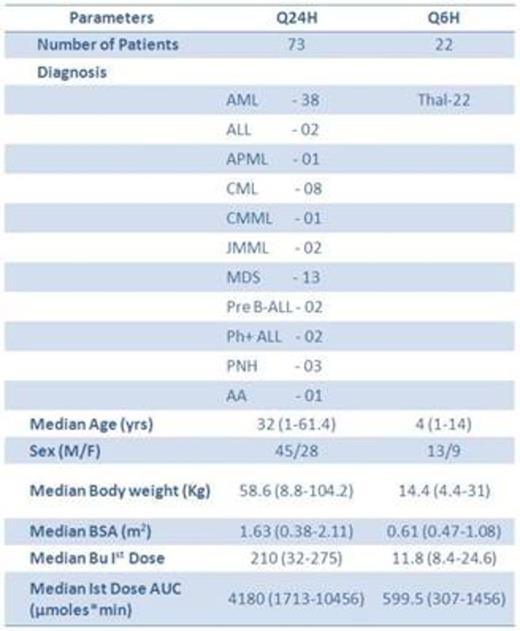
High-dose Busulfan (Bu) in combination with cyclophosphamide or fludarabine is widely used conditioning regimen prior to hematopoietic stem cell transplantation (HSCT) for various hematological malignant and non-malignant disorders. Intravenous (i.v) formulation of Bu, along with targeted dose adjustment has resulted in reliable systemic exposure (due to its predictable pharmacokinetics (PK)) while minimizing toxicity and treatment failure. We have previously demonstrated the population pharmacokinetics of oral Bu in thalassemia patients undergoing HSCT (Blood (ASH Annual Meeting Abstracts), Nov 2009; 114: 3349) and factors predicting or influencing the outcomes. Since 2010, a generic form of i.v Bu (BUCELON 60™, Celon Labs, India) with targeted dose adjustment has been used at our center, for HSCT with all Bu based conditioning regimen. There is no report on the PK of Bucelon in patients undergoing HSCT. The objectives of the present study were to evaluate the PK of Bucelon in patients undergoing HSCT, compare the systemic exposure to i.v Bucelon vs. previous reports in patients with various hematological diseases and report the toxicity and outcome after i.vBucelon in our patient cohort.
GSTM1 polymorphism on Bu AUC
When we analyzed the regimen related toxicity in these patients, 10 of the 95 (10.5%) developed sinusoidal obstruction syndrome (SOS) of which 3 was severe. Eight patients (8.4%) had not engrafted or rejected and 5 (5.2%) relapsed, 13 patients (13.7%) developed Grade III-IV GVHD and 3 had Hemorrhagic Cystitis. There was no significant difference in the cumulative AUC between those who develop toxicities and those who did not.
We report the PK of i.v Bucelon, a generic formulation of i.v Bu for the first time in patients undergoing HSCT. Since the systemic exposure to Bucelon seems to be lower in similar age, disease matched cohort, this may mean that targeted adjustment of Bu is necessary when we use i.vBucelon as conditioning regimen for HSCT. The lower systemic exposure could be due to population difference in PK or due to the drug formulation, or a combination of both, which needs to be validated further.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal