Abstract
Human Adenovirus (HAdV) reactivation is a frequent and potentially fatal complication in pediatric allogeneic stem cell transplantation (HSCT) recipients, especially after in vivo T-cell depletion. Patients with HAdV reactivations are generally treated with the antiviral agent Cidofovir (CDV), a monophosphate nucleotide analogue. Studies reporting effectiveness of CDV therapy are mostly incomplete due to lack of data on the immune status of the patients. Here, we report the relationship between the effectiveness of CDV therapy and lymphocyte reconstitution as well as the acute and chronic nephrotoxicity of CDV in the setting of pediatric .infections after pediatric HSCT.
Between 2003 and 2012, 321 children received 363 allogeneic transplantations in our pediatric HSCT center. HAdV reactivations were monitored by weekly plasma HAdV DNA RQ-PCR. Disseminated infections, defined as two consecutive PCR values above 103 (3 log ) copies / mL occurred in 40 patients (12 %). 30 patients were treated with CDV for infections with an HAdV load ≥ 3 log at start of therapy. In 27 patients, sufficient data were available to evaluate the effectiveness of CDV. Cidofovir was administered IV 3x / week at 1 mg / kg. Prehydration and Probenecid were used to reduce the risk of nephrotoxicity. Patient were median 4.5 (0.5 - 18) years of age and 26 of 27 patients received in vivo T-cell depletion. CDV therapy was initiated median 28 (1 - 121) days after HSCT while the median treatment duration was 15 (1 - 99) days. At start of CDV treatment, the HAdV load was median 4.2 log (range 3.1 log - 6.5 log) copies / mL.
Response was evaluated after 14 days of CDV therapy. A ≥ 10x (1 log) reduction of HAdV load was measured in 10 of 27 evaluable patients (37%) while 13 patients (48%) showed a stabilization of HAdV load. CDV treatment failed in 4 patients (15 %), as define by a 1 log increase of HAdV load despite uninterrupted CDV therapy. In 23 patients with a reduction or stabilization of the HAdV load, lymphocyte numbers increased strongly in the two weeks after initiation of CDV treatment (median 130 to 608 lymphocytes / µL, p < 0.0001, Wilcoxon signed-rank test). This increase was caused by reconstitution of NK cells (n = 5), T-cells (n = 4) or both (n = 12). In only 2 patients with a stabilization of HAdV load, no concomitant reconstitution of NK or T-cells was observed.
Ultimately, 17 patients (63 %) cleared the virus 5 - 89 (median 33) days after the initiation of CDV therapy. The moment of HAdV clearance was strongly correlated to the first day the T-lymphocytes reached 50 cells/ µl (R2 = 0.79, p < 0.0001, linear regression analysis). 7 patients died of progressive HAdV viremia with multi-organ failure (MOF) at 20 - 154 (median 51) days after initiation of CDV therapy. Three patients were not evaluable as they died of another cause (n=2) or were lost to follow up (n=1) without clearance of the virus.
For the analysis of CDV nephrotoxicity, 3 patients with HAdV related MOF during CDV therapy were excluded, leaving 24 patients. In 4 patients (17%), CDV therapy had to be discontinued because of acute glomerular failure, which invariably occurred within the first 2 weeks of treatment. Tubular kidney failure, defined by the need for suppletion of phosphate, magnesium or bicarbonate, was observed in 17 of 24 patients (71%). Kidney failure was transient in the majority of patients. After 1 year, 1 of 12 survivors (8 %) had chronic glomerular and tubular kidney failure that could not be explained by nephrotoxic co-medication. In 9 additional HSCT recipients who received > 7 days of CDV therapy for non-disseminated HAdV infections, 1 of 8 surviving patients (13 %) had chronic kidney failure that was unlikely to be caused by other nephrotoxic medication.
In conclusion, a reduction of HAdV load in the first 2 weeks of therapy was always accompanied by lymphocyte recovery while Cidofovir could not prevent further dissemination in 4 of 27 patients. In most patients with a stabilization of the viremia, this could be attributed to both CDV as well as T- or NK-cell reconstitution whereas in only 2 patients (7%), the HAdV load stabilized in the absence of lymphocyte recovery. These data suggest that CDV possibly plays a role in the stabilization of HAdV viremia in the time pending T-cell recovery. In view of the considerable toxicity profile, a multicenter randomized clinical trial is warranted to establish efficacy of CDV treatment for disseminated HAdV infections after pediatric HSCT.
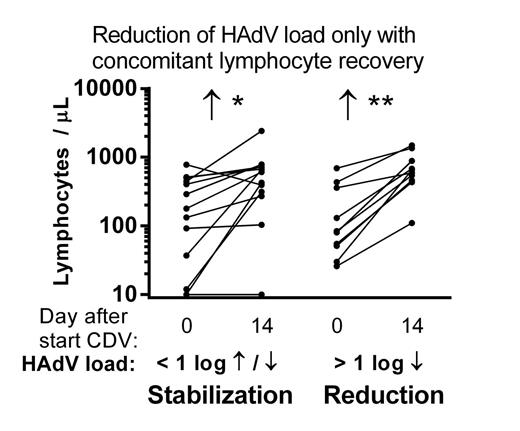
Off Label Use: The antiviral agent Cidofovir (CDV), a monophosphate nucleotide analogue, is widely used off-label for the treatment of disseminated Human Adenovirus infections after Pediatric Hematopoietic Stem Cell Tansplantation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal