Abstract
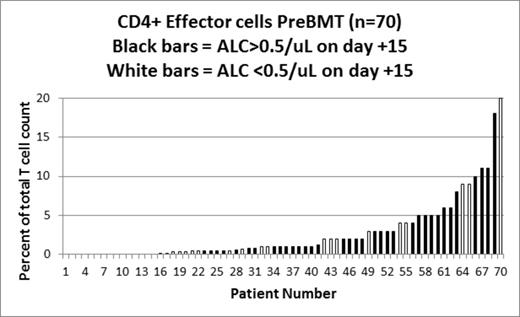
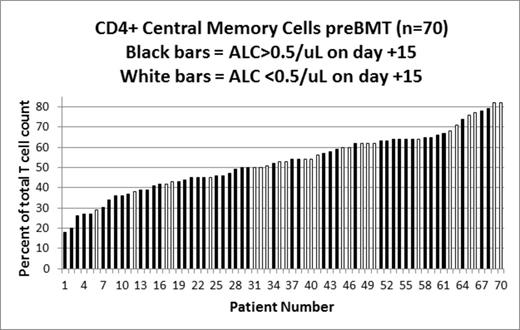
The treatment of transplant-eligible, multiple myeloma (MM) patients usually consists of induction therapy followed by autologous hematopoietic stem cell transplant (ASCT). In addition to typical cytogenetic and biochemical prognostic factors, there are other risk factors that predict for relapse and progression after ASCT. This includes absolute lymphocyte count (ALC) recovery (>0.5 cells/μL) by day +15 post ASCT, which has previously been shown to correlate with prolonged progression-free (PFS) and overall survival (OS). In a cohort of 70 MM patients who received high dose melphalan with ASCT between 8/2007 and 9/2012, we performed a comprehensive immunophenotyping panel including 35 T-, B-, NK- and Dendritic Cell (DC)-cell subsets a median of 25 days before ASCT (N=70), at day +30 post-ASCT (N=40) and at day +100 (N=51) post-ASCT. Specifically, the immunophenotyping panel included total numbers of T cells (CD3+, CD4+, CD8+), B cells (CD19+, CD20+), NK cells (CD56+CD16+) and myeloid (CD11c+) and plasmacytoid (CD123+) dendritic cells. T cell subsets included the relative proportions of double positive (CD3+CD4+CD8+), double negative (CD3+CD4-CD8-), CD4+ and CD8+ Naïve (CD3+ CD45RA+ CD45RO- CD27+), CD4+ and CD8+ central memory (CD3+ CD45RA- CD45RO+ CD27+), CD4+ and CD8+ effector memory (CD3+ CD45RA- CD45RO+ CD27-), recent thymic emigrants (CD3+ CD4+ CD45RA+ CD31+), and T-regulatory cells (CD3+ CD4+ CD25(br), CD3+CD4+CD25+CD127(d), CD3+CD4+CD25+CD127-HLADr+). B cell subsets included proportions of naïve (CD19+ CD27-), and memory (CD19+ CD27+, pre-switch CD19+ IgD+ CD27+, post-switch CD19+ IgD- CD27) cells. All patients survived to day +100. We examined MM response to ASCT, recovery of ALC by day +15 post-ASCT, clinically significant infections, PFS and OS. Characteristics in 70 patients pre-ASCT were: median (range) age 60 (28-75) yrs; 54% females; 26% KPS ≥90; response to most recent therapy was 13% in CR/sCR, 40% in VGPR, 41% in PR, 6% stable disease. MM responses at day + 100 included: 51% achieved or maintained CR/sCR, 17% VGPR, 10% PR, 24% SD and 6% progressed. The frequency of different immune cell subsets at either pre-ASCT or day +100 post-ASCT were not associated with CR/sCR status at day 100 post-AHSCT. ALC recovery by day +15 post-ASCT was associated with significantly longer median PFS (651 vs 288 days, P=0.001) and OS (804 vs 391 days, P=0.02) than those who did not recover ALC by day 15. For patients showing early recovery of ALC, the absolute numbers of T-cells pre-ASCT (P=0.08), as well as the proportion of CD4+ naïve (P=0.07) and CD8+ NK (CD8+/CD16+/CD56+, P=0.06) populations tended to be higher, whereas the absolute counts of gamma-delta T cells (P=0.02), CD4+ helper (P=0.03, See Figure), CD8+ effector (P=0.02) T-cells and total B-cell count (P=0.05) were significantly higher as compared to patients who did not achieve ALC recovery by day +15. Of note, within the T-cell compartment, CD4+ central memory (P=0.02, see Figure), CD8+ central memory (P=0.05), T-regulatory (T reg) (P=0.02, CD4+25+) populations were significantly lower. The distribution of different immune cell subsets pre-ASCT was not associated with development of clinically significant infections before day +100 post-AHCT; however, the proportion of memory B cells measured at day+100 was significantly higher (p=0.02) in patients who had experienced an infection before day +100. In the present study we show that the distribution of different immune cell populations correlates with ALC recovery in MM. Moreover, we also confirmed the association of ALC recovery with prolonged PFS and OS. The increase in the relative proportions of CD4+ and CD8+ effector cell subpopulations and the decrease in CD4+ and CD8+ central memory and T-reg populations demonstrate that the pre-ASCT effector cell phenotype contributes to faster ALC recovery, and improved PFS/OS. Therapeutic strategies to alter the pre-ASCT and/or day + 100 immune phenotype may improve outcomes after ASCT.
Figure 1
Figure 2
Disclosures:
McCarthy:Janssen: Honoraria; Celgene: Consultancy.
Topics:
autologous hematopoietic stem cell transplant with purging,
autologous hematopoietic stem cell transplant without purging,
lymphocyte count measurement,
multiple myeloma,
progression-free survival,
autologous stem cell transplant,
antigens, cd27,
infections,
immunophenotyping,
antigens, cd25
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal