Abstract
The role of high-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) in the treatment of multiple myeloma (MM) continues to evolve in with the availability of effective novel agents. It has been shown that exposure to novel agents (NA) can improve the post-relapse survival (Venner CP, et al, 2011). However, the impact of early relapse (ER) after ASCT in the era of NA has not been fully defined. In this study, we aimed to assess the impact of ER after ASCT on overall survival (OS) for MM patients (pts) undergoing single ASCT who had received NA induction regimens
All consecutive pts with documented MM undergoing single ASCT at Princess Margaret Cancer Center from 01/02 to 09/12 who had novel induction therapy were evaluated. ER was defined as progression of myeloma within 12 months from the ASCT. The Cox proportional hazard model was used to perform multivariate analyses of possible prognostic variables for overall survival. A p value of <0.05 was considered statistically significant.
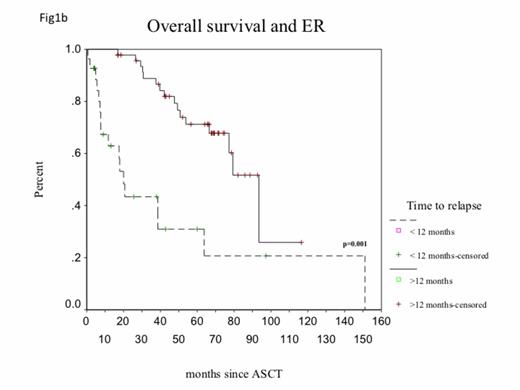
The clinical and laboratory characteristics are of the 184 pts given induction therapy with NAs are listed in Table 1. The median age for this cohort of patients was 58 years (range 31-72). At day-100 post-ASCT, CR was achieved in 15.3% and VGPR in 57.1%. At the time of this analysis, 81% of pts are alive and 75 (40.7%) have already progressed. The median OS of the whole group was 93 months (95% CI, 70-116) (Fig.1a ) and medianPFS was 25.43 months. Median time to relapse was 17.23 months (C I95%; 12-22). Early relapse was seen in 27/75 pts (36% of relapsed cases). Median OS was significantly shorter for the group of patients with ER (20 months, CI 95%; 15.56-24.51 versus 93 months, CI 95%; 75-111) (p=0.001) (Fig. 1b) Multivariate analysis showed the presence of ER as the major independent prognostic factor for OS; age > 60, B2M > 460 µmol/L, LDH > 350 IU/L, CRP > 20mg/L, albumin < 35g/L, and creatinine>200 µmol/L did not correlate with OS. In addition, FISH was available in only 78/184 cases. Only 1/27 pts in the ER group exhibited high-risk cytogenetics versus 0/48 in the non-ER group.
Clinical characteristics of patients with Multiple Myeloma treated with single ASCT after induction with novel agents containing regimens
| Clinical characteristic N=184 . | Median . | Range . | % . |
|---|---|---|---|
| Age (years) | 58 | 31-72 | |
| Male Female | 61.4% 38.6% | ||
| Hemoglobin (g/L) | 111 | 67-160 | |
| Creatinine (µmol/L) | 120 | 41-832 | |
| Calcium (µmol/L) | 2.3 | 1.94-3.30 | |
| B2-microglobulin ((µmol/L) | 190 | 129-909 | |
| Albumin (g/L) | 37 | 28-50 | |
| Heavy chain at diagnosis IgG IgA IgD Biclonal Light Chain only | 45.4% 25% 2.6% 6.0% 21% | ||
| Induction -CyBORD -Lenalidomide and Dexamethasone -Thalidomide plus Dexamethasone -Doxil, Velcade and Dexamethasone -Velcade and Dexamethasone | 48.9% 8.2% 27.2% 8.7% 8.7% |
| Clinical characteristic N=184 . | Median . | Range . | % . |
|---|---|---|---|
| Age (years) | 58 | 31-72 | |
| Male Female | 61.4% 38.6% | ||
| Hemoglobin (g/L) | 111 | 67-160 | |
| Creatinine (µmol/L) | 120 | 41-832 | |
| Calcium (µmol/L) | 2.3 | 1.94-3.30 | |
| B2-microglobulin ((µmol/L) | 190 | 129-909 | |
| Albumin (g/L) | 37 | 28-50 | |
| Heavy chain at diagnosis IgG IgA IgD Biclonal Light Chain only | 45.4% 25% 2.6% 6.0% 21% | ||
| Induction -CyBORD -Lenalidomide and Dexamethasone -Thalidomide plus Dexamethasone -Doxil, Velcade and Dexamethasone -Velcade and Dexamethasone | 48.9% 8.2% 27.2% 8.7% 8.7% |
*CyBORD: Cyclophosphamide, Bortezomib and Dexamethasone
Overall survival in the group of patients who underwent ASCT after novel agents induction therapy
Overall survival in the group of patients who underwent ASCT after novel agents induction therapy
Overall survival in the group of patients who underwent ASCT after novel agents induction therapy according to the type of relapse
Overall survival in the group of patients who underwent ASCT after novel agents induction therapy according to the type of relapse
In conclusion, not only the quality of response but also maintatenance of a deep response after ASCT, appear to be major prognostic variables in MM. Patients with ER post-ASCT should be biologically characterized to better understand the mechanisms of resistance associated with this particular entity and to develop new predictive models that can identify prospectively this myeloma subset. Also, patients with ER should be included in the definition of “high-risk” disease and priority candidates for the development of individualized therapeutic measures.
Reece:Otsuka: Honoraria, Research Funding; BMS: Research Funding; Merck: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Millennium Pharmaceuticals: Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Onyx: Consultancy. Chen:Roche: Honoraria; Johnson & Johnson: Consultancy, Research Funding; Lundbeck: Consultancy; Celgene: Consultancy, Research Funding; GlaxoSmithKline: Research Funding. Tiedemann:Celgene: Honoraria; Janssen: Honoraria. Kukreti:Millennium Pharmaceuticals: Research Funding; Onyx: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal