Abstract
The inhibition of oncogenic BCR-ABL1 kinase with tyrosine kinase inhibitors (TKIs) has significantly improved the prognosis of CML. Recent reports suggest that approximately 40 % of CML patients who have achieved optimal therapy response (complete molecular remission, CMR) can stop imatinib treatment without recurrence of detectable BCR-ABL1 transcripts. However, no predictive prognostic factors for successful therapy discontinuation have yet been identified. We therefore set up an immunological substudy in the ongoing pan-European EURO-SKI stopping study. We aimed to identify predictive biomarkers for relapse and non-relapse after TKI discontinuation. In addition, we aimed to understand more on the mechanisms of immune surveillance in CML and to study the effects of TKI treatment on the immune system.
Patients in deep molecular remission (MR4, BCR-ABL < 0,01% IS) for at least one year and with TKI treatment for at least 3 years were eligible for the clinical study. Basic lymphocyte immunophenotyping (the proportions and absolute numbers of NK-, T- and B-cells) was performed at the university hospital laboratories at the time of therapy discontinuation, and 1, 6, and 12 months after the TKI discontinuation. In a proportion of patients a more detailed immunophenotypic (analysis of CD45RA, CD57, CD27 and CD62L expressions) and functional analyses were done from fresh blood samples in a central immunology laboratory (Helsinki) at the same time points. The cytotoxicity of NK-cells was studied by measuring the direct killing of target cells (K562) and by the degranulation assay (CD107a/b expression). The secretion of Th1 type of cytokines IFN-γ/TNF-α was studied from both T- and NK-cells.
Thus far the basic lymphocyte subclass measurement has been analyzed from 62 patients who have discontinued TKI treatment within the EURO-SKI study. Functional analyses have been performed from 30 patients. 60 patients have used imatinib before treatment discontinuation and 2 patients dasatinib.
At baseline, before the treatment discontinuation both CD4+ and CD8+ T-cell counts were within the normal range (median CD4+ 0.73x 109/L, range 0.11-2.4x 109/L; CD8+ 0.35x 109/L, 0.07-1.92 x 109/L). The TKI stop had no significant numerical or functional effect on T-cells, and at 1 month time-point the median T-cell counts were unchanged (CD4+ 0.73x 109/L; CD8+ 0.35x 109/L). Similarly, at the baseline, the median NK-cell count was within a normal range (0.26 x 109/L, range 0.04-1.04 x 109/L), and no significant change was observed 1 month after stopping the treatment (median 0.29 x 109/L). Furthermore, at the baseline and at the 1-month time-point the cytotoxicity of NK-cells and the cytokine secretion of T- and NK-cells did not significantly differ from the healthy controls when all patients were considered as a one group.
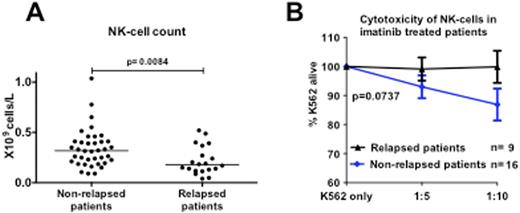
However, when patients were divided in two groups based on the relapse status, the patients who eventually relapsed had significantly fewer NK-cells already at the baseline (Figure A; absolute count 0.18x 109/L vs. 0.32 109/L, p=0.008; proportions 11% vs. 21%, p=0.001). The phenotype of NK-cells also differed between the two groups, and the patients who relapsed had less NK-cells expressing CD57 (median 58% vs. 69%, p=0.046) and CD16 (median 67% vs. 83%, p=0.018) on the cell surface. Furthermore, the cytotoxicity of NK-cells was impaired in patients who failed to discontinue the TKI treatment successfully and no killing activity was observed in their samples (Figure B; alive K652 cells after co-incubation with effector cells 100% vs. 88%, p=0.07). No clear differences were observed in the function or the numbers of T-cells between relapsing and non-relapsing patients.
Numbers and function of NK-cells at baseline. A) Absolute NK-cell counts B) Cytotoxicity of NK-cells after a 6-hour co-incubation with target cells (K562).
Numbers and function of NK-cells at baseline. A) Absolute NK-cell counts B) Cytotoxicity of NK-cells after a 6-hour co-incubation with target cells (K562).
The NK-cell numbers and their function may predict disease relapse after TKI discontinuation. This may have impact on the future stopping trials. In addition, it further illustrates the importance of the immune system in the successful long-term treatment of CML.
Ekblom:Novartis: Honoraria; Bristol-Myers Squibb: Honoraria. Hjorth-Hansen:Pfizer, BMS: Honoraria, Travel expenses Other. Porkka:BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Richter:Bristol-Myers Squibb: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Mustjoki:Novartis: Honoraria; BMS: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal