Abstract
Limited effect of JAK2 inhibitors in treating myelofibrosis (MF), either primary (PM) or secondary to polycythemia vera (PV-MF) or essential thrombocythemia (ET-MF), and the limited survival experienced with stem cell transplantation, prompted us to use interferon (rIFNα) in early stage MF (eMF), i.e. low or intermediate-1 disease. rIFNα effects megakaryopoiesis, decreasing megakaryocyte (MK) density and size, inhibiting thrombopoietin-induced signaling, and reducing levels of platelet derived growth factor, which play a major role in the pathogenesis of MF. Moreover, rIFNα affects stem cells in PV. Results we previously reported in eMF showed remission, clinical improvement (CI) or stable disease (SD) in 80% of 17 patients (pts.) with PM (Silver RT et al. Blood. 2011; 117(24):6669-72). Based on this encouraging experience, we expanded our trial with rIFNα to include PV-MF and ET-MF. We now report the results of 32 pts. with eMF.
Pts. were seen regularly in our clinic, evaluating symptoms, physical findings, signs of toxicity, routine blood counts and chemistries and JAK2 determinations. Bone marrow biopsies (BMBs) were encouraged annually to evaluate status of disease. The dose of recombinant interferon alpha-2b (rIFNα-2b) treatment was initiated at 500,000 – 1,000,000 units thrice weekly (18) or pegylated interferon alpha-2a (PEG-IFNα-2a), 45 mcg weekly (9).
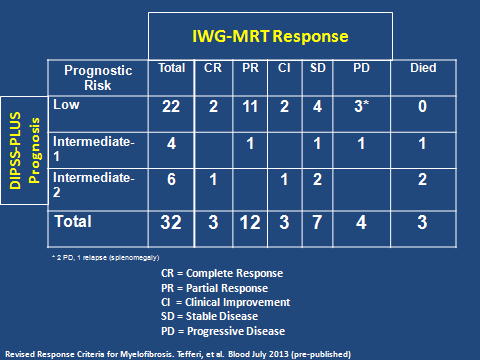
17 women and 15 men, median age 57 years (range: 34-72) were treated. Median duration of disease prior to treatment was 3.4 months. Median values for hematocrit, hemoglobin, and platelet count at baseline were 38.8%, 12.9 g/dL, and 345,000/uL, respectively. 12 pts. (37.5%) met the criteria for PM, 7 (22%) for PV-MF and 11 (34%) for ET-MF. Two (6.3%) PV pts. had continuing phlebotomy requirements and progressive splenomegaly, and marrow fibrosis in the absence of other WHO criteria. Risk categories were assigned based on the IWG-MRT DIPSS-PLUS. Twenty-two pts. were low-risk, 4 intermediate-1, and 6 intermediate-2 (the latter group emerged solely due to incorporation of cytogenetic data; prior to DIPSS-PLUS, the 6 pts. categorized as intermediate-1). The IWG-MRT risk categories were correlated with response (Table). Of 32 pts., 3 (9.4%) had complete remission (CR), 12 (37.5%) partial remission (PR), 3 (9.4%) CI and 7 (22%) SD - an overall response rate of 78%. Three (9.4%) pts. (1: intermediate-1, 2: intermediate-2) died of progressive disease (PD), one of whom was rIFNα non-compliant. Of 32 pts., 10 had no splenomegaly at onset of rIFNα, and 9 remained unchanged during treatment. In 10 pts. initially with slight splenomegaly (< 3 cm below the LCM), the spleen became non-palpable in 8. Of 3 pts. with moderate (>3 – 9 cm BLCM) spleens, and 9 (> 9 cm BLCM) with marked splenomegaly, there was a more variable response (1 – 16 cm decrease). Follow-up BMBs were possible in 22 pts.; in 12, reduction in cellularity occurred after a median treatment period of 2 years. Of 15 pts. with reduction in splenomegaly and evaluable BMBs, 7 had reduction in cellularity, 3 showed significantly improved MK morphology, marrow architecture, and striking reduction of reticulin and collagen fibrosis (grade 3 to 1); 3 showed no change in morphology, but nevertheless, experienced reduction in spleen size. Of the 32 pts., 23 (71.8%) were JAK2V617F (+). Of 19 follow-up specimens, 7 had a median allelic burden decrease of 44%., the remainder were unchanged Abnormal cytogenetics did not influence response.
All pts. initially experienced the usual side effects of rIFNα which were mild in degree (grade 1-2) and subsided with time or dose adjustment; including mild depression, dry skin, cough, myalgia, fatigue and asthenia. Seventeen pts. developed increasing anemia (13: grade 1; 4: grade 2); one had grade 3 thrombocytopenia. In these cases, the dose was temporarily reduced or interrupted until cytopenias resolved. One pt. developed hyperthyroidism and treatment was discontinued.
In this eMF, single-arm study, we conclude that rIFNα prevents disease progression and can induce remission with acceptable toxicity, improving marrow morphology which usually, but not always, correlates with reduction in splenomegaly, warranting an expanded phase 3 trial, currently underway.
IWG-MRT treatment response stratified by DIPSS-PLUS risk at baseline.
Silver:Sanofi: Consultancy; Sanofi: Research Funding; Sanofi: Honoraria. Off Label Use: The use of recombinant and pegylated interferon alpha in the treatment of myelofibrosis. Feldman:Tragara Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal