Abstract
Fedratinib (SAR302503), a JAK2-selective inhibitor, has demonstrated clinical improvements in splenomegaly and constitutional symptoms in patients with MF in Phase I/II trials (J Clin Oncol 2011;29:789. Haematologica 2013;98:S1113). The aim of this primary analysis was to determine the effect of fedratinib on key MF symptom burden and global assessment of HRQoL in the JAKARTA trial (NCT01437787).
JAKARTA is a double-blind, placebo-controlled, international, 3-arm, Phase III study, in which patients ≥18 years of age with intermediate- or high-risk MF, platelet count ≥50 × 109/L, and splenomegaly were randomized (1:1:1) to receive placebo or fedratinib at a dose of 400 or 500 mg, orally, once daily, in consecutive 4-week cycles. Total symptom score (TSS), a key efficacy end point (TSS: averaged daily total score of 6 item measures over 1 week: night sweats, pruritus, abdominal discomfort, early satiety, pain under ribs on left side, and bone or muscle pain), was assessed through a daily electronic eDiary using the modified Myelofibrosis Symptom Assessment Form (MFSAF; Cancer 2011;117:4869. Blood 2011;118:401), with symptom response defined as a ≥50% reduction in TSS at the end of Cycle 6 (EOC6). HRQoL was assessed using the EuroQOL (EQ)-5D instrument that was completed at baseline and EOC6. Patient performance was assessed using the Eastern Cooperative Oncology Group Performance Scale (ECOG PS). Spleen volume was measured by MRI or CT at baseline and EOC6.
In JAKARTA, a total of 289 patients were randomized: median age 65 years; 59% male; 63% primary MF; 48% high-risk MF; 67% JAK2V617F positive; 16% platelet count <100 × 109/L. The symptom evaluable population comprised 261 patients (placebo [n=82]; 400 mg [n=89]; 500 mg [n=90]). The mean (SD) baseline TSS was 14.6 (11.9), 17.6, (13.5), and 16.9 (11.9) in the placebo, 400 mg, and 500 mg groups, respectively. At Week 24 (EOC6), the proportion of patients with a symptom response was significantly higher (p<0.0001) in the 400 and 500 mg groups versus placebo (Table). Symptom responses with fedratinib were also higher than placebo in the subgroup of patients with baseline platelets <100 × 109/L (Table). For individual symptoms, the greatest improvements were seen for night sweats and early satiety (Table). Baseline HRQoL (EQ-5D, mean [SD]) was similar in the three groups (placebo: 62.5 [21.2]; 400 mg: 61.3 [22.2]; 500 mg: 60.1 [20.1]). Fedratinib treatment led to improvements in HRQoL from baseline to Week 24, whereas placebo treatment led to slight worsening of HRQoL (Table).
Table. Symptom response and HRQoL
| . | Placebo (n=82) . | Fedratinib 400 mg (n=89) . | Fedratinib 500 mg (n=90) . |
|---|---|---|---|
| Total symptom responsea at EOC6, n (%) | 6 (7.3) | 33 (37.1)* | 31 (34.4)* |
| Patients with baseline platelets <100 × 109/L, n/N (%) | 0/16 (0) | 3/13 (23.1) | 3/15 (20.0) |
| Patients with baseline platelets ≥100 × 109/L, n/N (%) | 6/66 (9.1) | 30/76 (39.5) | 28/75 (37.3) |
| Median change from baseline at EOC6 for individual symptoms (%) | |||
| Night sweats | –23.5 | –84.2 | –100.0 |
| Early satiety | 6.4 | –67.5 | –64.5 |
| Pain under ribs on left hand side | –7.3 | –61.3 | –60.0 |
| Pruritus | –28.6 | –52.1 | –51.0 |
| Abdominal discomfort | 2.8 | –50.0 | –53.9 |
| Bone or muscle pain | 8.3 | –27.7 | –28.2 |
| EQ-5D change from baseline at EOC6, mean (SD) | -1.2 (24.1) | +6.8 (18.7) | +3.2 (17.2) |
| 95% CI | -7.8; 5.4 | 2.3; 11.2 | -1.4; 7.8 |
| Difference from placebo | 8.0 | 4.4 | |
| . | Placebo (n=82) . | Fedratinib 400 mg (n=89) . | Fedratinib 500 mg (n=90) . |
|---|---|---|---|
| Total symptom responsea at EOC6, n (%) | 6 (7.3) | 33 (37.1)* | 31 (34.4)* |
| Patients with baseline platelets <100 × 109/L, n/N (%) | 0/16 (0) | 3/13 (23.1) | 3/15 (20.0) |
| Patients with baseline platelets ≥100 × 109/L, n/N (%) | 6/66 (9.1) | 30/76 (39.5) | 28/75 (37.3) |
| Median change from baseline at EOC6 for individual symptoms (%) | |||
| Night sweats | –23.5 | –84.2 | –100.0 |
| Early satiety | 6.4 | –67.5 | –64.5 |
| Pain under ribs on left hand side | –7.3 | –61.3 | –60.0 |
| Pruritus | –28.6 | –52.1 | –51.0 |
| Abdominal discomfort | 2.8 | –50.0 | –53.9 |
| Bone or muscle pain | 8.3 | –27.7 | –28.2 |
| EQ-5D change from baseline at EOC6, mean (SD) | -1.2 (24.1) | +6.8 (18.7) | +3.2 (17.2) |
| 95% CI | -7.8; 5.4 | 2.3; 11.2 | -1.4; 7.8 |
| Difference from placebo | 8.0 | 4.4 | |
Proportion of patients with ≥50% reduction in TSS from baseline.
Significantly different versus placebo (p<0.001).
At Week 24, the degree of improvement in TSS was greatest in patients with ≥35% reduction in spleen volume from baseline (Figure). Mean TSS improvement was correlated with improvement in HRQoL (TSS reductions were greater in EQ-5D improvers versus non-improvers), and ECOG PS (TSS reductions were greater in patients with ECOG PS 1 or 2 score improvement versus those with ECOG PS worsening).
Mean change in TSS by spleen volume reduction (all patients)
Mean change in TSS by spleen volume reduction (all patients)
Fedratinib treatment over 24 weeks led to significant improvements in MF symptoms versus placebo. Patients treated with fedratinib also experienced substantial improvements in HRQoL versus placebo. Symptom improvements were associated with spleen responses. This study was sponsored by Sanofi.
Mesa:Incyte, Genentech, Lilly, MS Pharma, Gilead: Research Funding. Cortes:Incyte, Sanofi: Consultancy; Incyte, Sanofi: Research Funding. Cervantes:Novartis: Speakers Bureau; Novartis and Sanofi: Membership on an entity’s Board of Directors or advisory committees. Jourdan:Sanofi: Honoraria. Vannucchi:Novartis: Membership on an entity’s Board of Directors or advisory committees. Drummond:TargeGen, Novartis: Speakers Bureau; Sanofi, Novartis, Celgene: Honoraria; Sanofi, Novartis, Celgene: Consultancy. Passamonti:Novartis, Celgene, Incyte, Sanofi, Roche: Honoraria. Neumann:Sanofi: Employment. Joulain:Sanofi: Employment. Iqbal:Sanofi: Employment. Harrison:Novartis: Research Funding; Novartis, Sanofi, YM Bioscience, Celgene, SBio, Gilead: Honoraria; Novartis, Sanofi, Shire: Speakers Bureau; Novartis, Sanofi, YM Bioscience, SBio, Gilead: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

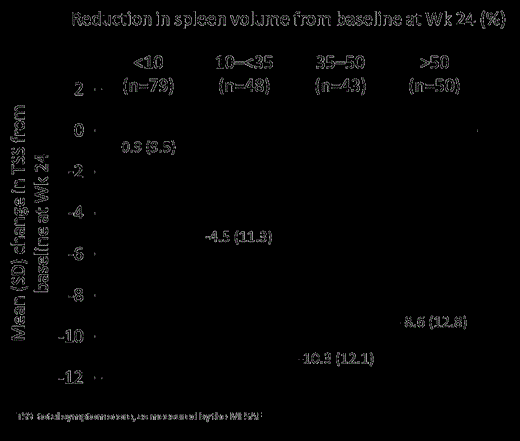
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal