Abstract
While the utility of positron emission tomography (PET) compared with computed tomography (CT) for end-of–induction (EOI) therapy response assessment in follicular lymphoma (FL) remains unclear, emerging data suggest that PET performed at the end of therapy can predict survival. To further define the role of PET compared with CT in the management of patients with FL, we used the National LymphoCare Study (NLCS) database to examine the use of PET and CT in clinical practice, to assess the prognostic role of PET and CT after induction therapy, and to evaluate whether PET provides better prediction of outcomes compared with response based on CT scans.
NLCS is an observational study comprising 2700+ FL patients enrolled between 2004 and 2007. In NLCS, 1072 patients with FL completed induction rituximab (R) monotherapy or R-chemotherapy and had EOI imaging response assessments via PET ± CT or CT alone performed between 2 cycles prior to and 12 weeks after the end of therapy. Response assessments (complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD]) were determined by the local investigators; CR was classified as a negative scan, while PR, SD, and PD were classified as positive scans. Multivariate logistic regression was used to evaluate baseline factors associated with receiving PET imaging. Outcomes were defined as the number of days from the EOI response assessment until date of death (overall survival [OS]), date of disease progression (as determined by the treating physician) or death (progression-free survival [PFS] defined for patients without PD at date of EOI assessment). To directly compare survival in each imaging group, a propensity score (PS) was calculated to adjust for imbalances between the groups. Cox proportional hazards models with PS matching were used to estimate the effects of PET and CT response on OS and PFS. All variables potentially related to outcome or imaging selection were included in the calculation of the PS. A total of 395 and 380 matched pairs were available for comparative analysis of OS and PFS, respectively. Kaplan-Meier estimates of PFS and OS were also calculated.
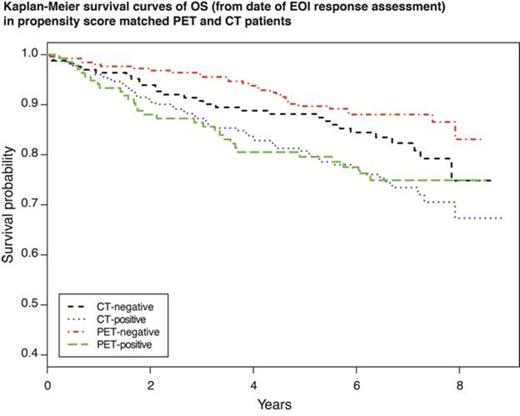
Of 497 PET ± CT scans performed at EOI, 330 (66.4%) were reported as negative, and 167 (33.6%) were reported as positive. Of 575 CT scans performed at EOI, 233 (40.5%) were reported as negative, and 342 (59.5%) were reported as positive. Grade 3 histology, available bone marrow assessment, Southwest region, and R-CHOP induction were associated with greater likelihood of receiving PET imaging. Median follow-up was 6.3 years. Five-year PFS and OS outcomes are detailed in Table 1. Patients who remained PET-positive had significantly poorer OS (PS-adjusted hazard ratio [HR] 2.21, 95% confidence interval [CI] 1.32–3.68) and PFS (PS-adjusted HR 1.48, 95% CI 1.06–2.07) compared with patients who were PET-negative at EOI. Compared with patients who were CT-negative at EOI, patients with CT-positive scans at EOI trended toward inferior OS (PS-adjusted HR 1.50, 95% CI 0.96–2.34) and PFS (PS-adjusted HR 1.37, 95% CI 1.00–1.87) outcomes, but the trend was not statistically significant. Patients with PET-positive vs CT-positive scans had no significant differences in OS (PS-adjusted HR 0.96, 95% CI 0.61–1.51) and PFS (PS-adjusted HR 1.10, CI 95% 0.78–1.39) outcomes. Patients with PET-negative vs CT-negative scans had no significant differences in OS (PS-adjusted HR 0.65, 95% CI 0.39–1.08) and PFS (PS-adjusted HR 1.02, 95% 0.75–1.39) outcomes.
Five-year PFS and OS (%)
| . | PFS . | OS . |
|---|---|---|
| PET-negative | 66.5 | 89.6 |
| PET-positive | 52.1 | 79.8 |
| CT-negative | 66.2 | 87.8 |
| CT-positive | 54.0 | 78.5 |
| . | PFS . | OS . |
|---|---|---|
| PET-negative | 66.5 | 89.6 |
| PET-positive | 52.1 | 79.8 |
| CT-negative | 66.2 | 87.8 |
| CT-positive | 54.0 | 78.5 |
After accounting for baseline differences between patients receiving PET and CT response assessments, PET response performed after R-induction therapy is a prognosticator of OS and PFS in patients with FL, while CT response shows a trend toward association with OS and PFS, which is not statistically significant. There is a trend toward improved OS in PET-negative compared with CT-negative patients, but it is not statistically significant. There is no difference in PFS or OS when comparing PET-positive with CT-positive patients. PET performed at the end of R induction in patients with FL is highly predictive of outcome; however, it remains uncertain whether response by imaging with PET has better predictive power of survival compared with conventional imaging with CT.
Off Label Use: Review will likely involve off label use of drugs for follicular lymphoma in the upfront setting. Byrtek:Genentech: Employment, Equity Ownership. Flowers:Bio-Oncology: Consultancy; Genentech: Consultancy; Celgene: Consultancy; Janssen: Research Funding; Spectrum: Research Funding; Sanofi: Research Funding; Celgene: Research Funding; Abbott: Research Funding; Millennium/Takeda: Research Funding. Link:Millenium: Research Funding; Genentech: Research Funding; Spectrum: Consultancy; Pharmacyclics: Consultancy; Millenium: Consultancy; Genentech: Consultancy; Pharacyclics: Research Funding. Zelenetz:Cephalon: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Sanofi-Aventis : Consultancy; Genentech: Research Funding; GSK: Research Funding; Roche: Research Funding; Cancer Genetics: Scientific Advisor Other; Celgene: Consultancy; GSK: Consultancy. Dawson:Roche: Equity Ownership; Genentech: Employment. Reid:Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal