Stem cell rescue after myeloablative doses of beta particle emitting radiolabeled monoclonal antibodies targeting CD20 antigen can lead to remissions in up to 95% of lymphoma patients who previously failed conventional combination chemotherapy. While encouraging, toxicities with beta particle radioimmunotherapy (RIT) are significant and ∼50% of patients ultimately relapse. Higher doses of absorbed radiation to tumors have correlated with a reduced risk of disease recurrence but dose limiting toxicities prevent escalation. A potential advantage of alpha particle emitting radionuclides is the release of large amounts of energy linearly over a few cell diameters (∼50-80 mm) resulting in irreparable double-strand DNA breaks that overwhelm cellular repair mechanisms. As a result, alpha particles confer a unique capacity to kill individual targeted cells while causing minimal radiation damage to surrounding tissues.
To explore alpha-emitter conjugated anti-CD20 RIT, a closo-decaborate(2-) [B10] labeling reagent was developed as a radiolabeling platform capable of providing critical stability to alpha particle-labeled biomolecules. 211At was chosen as a therapeutic radionuclide based on its high linear energy transfer, biologically relevant t1/2 (∼7.2 hr) and absence of toxic daughter radionuclide decay byproducts. Cell binding assays using CD20-expressing Ramos tumor cells demonstrated that B10 conjugated to the anti-CD20 monoclonal antibody (mAb) 1F5 (B10-1F5) was specific and that astatination did not impair antibody binding (211At-B10-1F5 binding was equivalent to 125I-B10-1F5). Blood clearance was similar for 125I-1F5, 211At-B10-1F5 and 125I-B10-1F5 in athymic nude- Foxn1nu mice (23.87 ±5.13 % injected dose/gram [ID/g], 19.41 ±3.27 %ID/g and 19.80 ±1.44% ID/g respectively) at 17 hr post infusion. In tissue biodistribution studies using nude mice bearing Ramos flank tumor xenografts (10 x106 cells injected) radioactivity was measured in blood, tumor and nonspecific organs harvested 24 hours after injection (n=5/group). Animals received either 125I-1F5, 211At-B10-1F5 and 125I-B10-1F5 (co-injected) or isotype matched control mAb 211At- B10-HB8181 and125I-B10-HB8181(co-injected). Measured activity in tumors was three-fold higher for 125I-1F5, 125I-B10-1F5 and 211At-B10-1F5 (7.62 ± 2.09%ID/g, 7.53 ± 1.59%ID/g and 9.28 ± 1.85%ID/g respectively) than for 125I-B10-HB8181 and 211At-B10-HB8181 controls (2.87 ± 0.35%ID/g and 3.45 ± 0.58%ID/g respectively). In non-target organs no appreciable difference in measured activity was seen with either 211At- or 125I-labeled B10-1F5 and their respective controls.
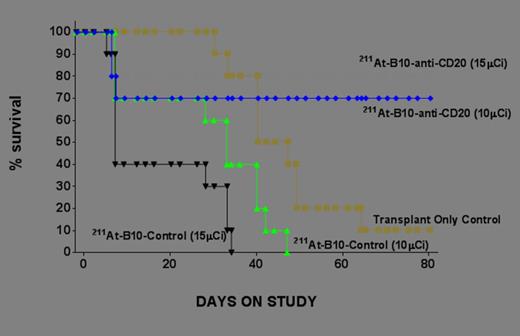
211At-B10-1F5 can eliminate CD20 expressing tumor cells in this mouse model and further study appears to be warranted.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal