We conducted a trial of bortezomib in patients with steroid-refractory cGVHD. Patients received 1.6 mg/m2 q wk x 4 followed by one week of rest, for up to six cycles, with some going on to a maintenance phase of treatment every other week for an additional 6 months. Twenty-one patients were registered on trial two patients withdrew before beginning treatment. Nineteen patients received between two and twelve months of treatment. The treatment was well tolerated with no grade 3 or higher adverse events (AE). Six patients had either CR or near CR. Average Rodnan scores on the 8 patients with sclerosis completing 2 or more cycles of therapy decreased from 22.6 ± 12.8 to 5.9 ± 6.2, p<0.005. One patient with non-healing suppurating lesions of his lower extremities had marked healing, another had significant decrease in chronic diarrhea. Weekly bortezomib for cGVHD was well tolerated and resulted in early improvement in a subset of patients with long standing refractory disease.
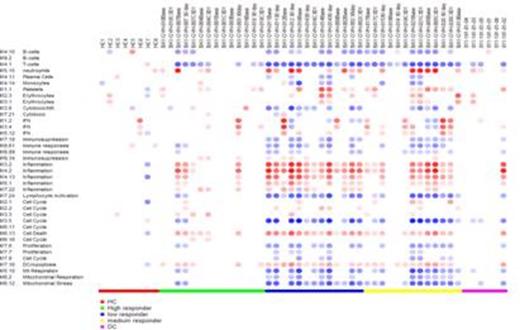
HC – Healthy Control; DC – Disease Control (allogeneic BMT recipients without cGVHD)
Weekly bortezomib for cGVHD appears to be well tolerated and results in early improvement in long standing refractory disease in a subset of patients. Gene expression profiles at the initiation of treatment correlated with response and may be useful in predicting which patients will respond to bortezomib.
Miller:Millennium Pharmaceuticals: Research Funding. Off Label Use: Bortezomib for chronic graft vs. host disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal