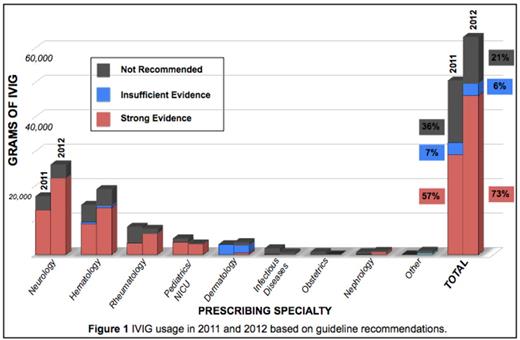
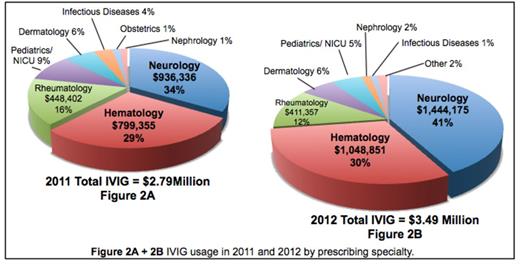
Intravenous Immunoglobulin (IVIG) is a plasma protein used in the treatment of various diseases. The cost of IVIG is approximately $50-$80 per gram. In 2005, Saskatchewan's usage of IVIG was above the national average, at 100g per 1000 persons compared to 95g per 1000 persons. IVIG is currently licensed by Health Canada for use in six diseases; however, IVIG is often used for off-label indications. There are several published guidelines that outline recommended uses of IVIG. These are largely based on case studies and expert opinion. Despite these guidelines, IVIG is often used in multiple settings where there is no supporting evidence.
We aimed to evaluate adherence to published guidelines by quantifying IVIG usage in the Saskatoon Health Region (SHR). We conducted a retrospective chart review of patients who received IVIG in 2011 and 2012, classifying the usage based on diagnosis and prescribing specialty. Our primary endpoint was the percentage of appropriate use of IVIG in comparison to published guidelines. Our secondary endpoint was adverse reactions seen in patients receiving IVIG.
In conclusion, our data analysis shows that IVIG usage in the SHR does not adhere well to guidelines. Pre-printed order sets outlining appropriate indications for IVIG are currently being introduced in the SHR to help improve adherence to guidelines. After the order sets have been implemented for one year, we will re-evaluate the usage of IVIG in the SHR to see if improvements have been made. Our goal is to ensure there is appropriate resource allocation of IVIG and to reduce risks to the patient and financial costs to the health care system.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal