Abstract
In vitro culture of adult human mesenchymal stem cells (hMSCs) can induce cancerous transformation, depending on environmental factors. To evaluate the passage dependent chromosomal changes of hMSCs toward malignant transformation, we passaged adipose origin hMSC to 9th passage and analyzed cytogenetic change, molecular cytogenetic changes, and telomere length variations on every passage.
On each passage, in situ karyotyping was performed on 3 separate batches with subsequent Giemsa staining. Karyotyping was analyzed using Metafer system (MetaSystems, Altlussheim, Germany). For analysis of nonproliferating interphase cell, each chromosome were counted with centromere fluorescent in situ hybridization (FISH) using Same Day OligoFISH™(Cellay Inc., Cambridge, Massachusetts, USA). Telomere length was analyzed using FISH technique with a Cy3-lableled Telomere peptide nucleic acid (PNA) FISH kit (DakoCytomation Denmark A/S, Glostrup, Denmark). To confirm the chromosomal translocation appeared by in situ karyotyping, we made home-brew FISH probe with bacterial artificial chromosome(BAC) clone and quantitated the proportion of abnormal cells by interphase FISH.
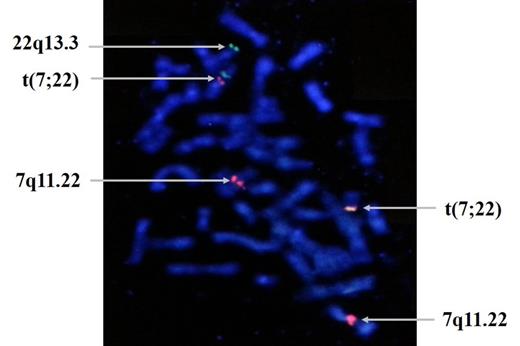
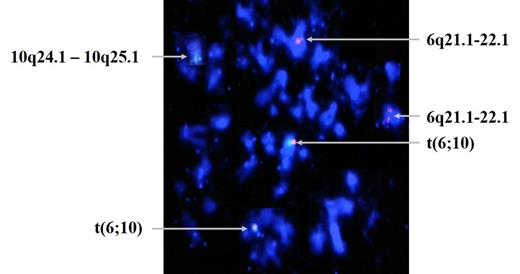
On 5th passage, translocation and polysomy of chromosome 7 and 9 appeared, and on 6th passage, additional translocation t(6; 10) appeared. ISCN Karyotypes of chromosomal changes from 5th passage to 7th passage were 48,XX,+7,t(7;22)(q11.22;q13.3),+9[4]/46,XX[21] → 47,XX,+7[2]/47,XX,t(6;10)(q21;q25.1),+7[2]/46,XX[13] → 48,XX,+7,t(7;22)(q11.22;q13.3),+9[6]/ 47,XX,+7[5]/46,XX[9]. Telomere length was decreased on late passages. Fusion signal of t(7;22) on passage 5(fig 1) and that of t(6;10) on passage 6(fig 2) were confirmed by BAC clone.
The behavior of late passage (from passage 5) follows a cytogenetic profile similar to that of transformed cancer cells. Cytogenetic abnormalities which were not observed in earlier passage, showed up and disappeared, but eventually persisted during passages. We suggest in vitro environment cause hMSCs to undergo cancer-like cytogenetic changes. It is of great importance to test safeguards for clinical applications of human stem cells manufactured in vitro.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal