Abstract
Despite gains in survival with aggressive upfront treatments, such as R-CHOP (rituximab, doxorubicin, vincristine, prednisone) for follicular lymphoma (FL), and the implementation of rituximab maintenance, approximately 20% of patients will still experience progression of disease (POD) within 24 months of chemoimmunotherapy, as demonstrated by the PRIMA study and others (Salles et al, Lancet 2010; Press et al, JCO 2013). This suggests a high-risk group of patients warranting further study. We analyzed the National LymphoCare Study to identify patients with FL who are at high risk of death after treatment with R-CHOP. We aimed to define what factors constitute high-risk FL that would merit further clinical and biological characterization.
Evaluable patients with stage II, III, and IV FL who were treated with R-CHOP in the first-line setting were included. Patients were excluded if the initial treatment strategy was watchful waiting or if there was POD prior to beginning first-line treatment. Two groups were defined: patients with POD/death ≤2 years from diagnosis (early POD group) and a reference group without POD/death within 2 years of diagnosis (Ref).
Multiple logistic regression compared baseline characteristics by group. Survival probability was estimated by the Kaplan-Meier method. A Cox model evaluated the effect of early POD on overall survival (OS). The Pearson chi-squared test and multiple logistic regression with backwards stepwise selection were used to evaluate whether baseline characteristics differed by group. OS was estimated using the Kaplan-Meier method, and a time-varying Cox model was used to evaluate the relationship between progression time and OS.
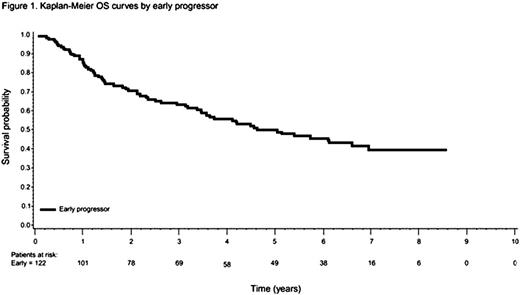
A total of 588 patients were analyzed. Similar to results from the PRIMA study, approximately 21% of patients (122/588) had early POD, while 71% (420/588) did not, and 8% (46/588) were lost to follow-up within 2 yrs. Early POD following R-CHOP was dramatically and significantly related to worsened OS, with a hazard ratio of 12.3 (95% confidence interval [CI] 6.1—24.9), compared with those not progressing. After adjusting for FLIPI, patients with early POD who were still alive within 2 years of R-CHOP (early POD—alive at 2 yrs) had worse OS compared with patients who did not progress within 2 years (Ref group) [HR = 4.76, 95% CI 2.95—7.69]. At 5 years, patients with early POD had OS of 50% compared with an OS of 95% in the Ref group (Table 1 and Figure 1). Multiple logistic regression analysis showed that high lactate dehydrogenase (LDH), poor Eastern Cooperative Oncology Group (ECOG) performance status, B symptoms, and bone marrow involvement were significantly associated with early POD (P<0.05). As an exploratory analysis, we evaluated maintenance and second-line treatments following R-CHOP. In the early POD group, 22% received maintenance rituximab, and 80% began second-line treatment, compared with the reference group, where 45% received maintenance rituximab, and 23% began second-line treatment. The impact of second-line autologous stem cell rescue or allogeneic transplant on survival is unknown because of the small numbers of patients in these groups.
Survival in patients with FL treated with first-line R-CHOP
| . | N . | 2-year OS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|
| Early progressors (POD) | 122 | 71% (61.5—78.0) | 50% (40.3—58.8) |
| Ref group (Late/no POD) | 420 (102/318) | 100% | 95% (92.7—97.0) |
| . | N . | 2-year OS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|
| Early progressors (POD) | 122 | 71% (61.5—78.0) | 50% (40.3—58.8) |
| Ref group (Late/no POD) | 420 (102/318) | 100% | 95% (92.7—97.0) |
Relapse of FL after first-line treatment with R-CHOP is associated with poor outcomes. However, POD within 2 years of R-CHOP defines a unique category of patients at substantially increased risk of death. In our unselected cohort of 588 patients, 63% (65/104) of deaths in 6 years of follow-up occurred in the early POD cohort. High LDH, poor ECOG performance status, B symptoms, and bone marrow involvement conferred a significantly high risk for early POD. These high-risk patients represent a distinct population that warrants further study in directed prospective studies of FL biology and clinical trials.
Byrtek:Genentech, a member of the Roche group: Employment, Stock ownership Other. Dawson:Genentech, a member of the Roche group: Employment, Stock ownership Other. Flowers:Abbott, Celgene, Millennium/Takeda, Sanofi, Spectrum, Janssen: Research Funding; Celgene, Genentech Bio-Oncology: Consultancy. Link:Genentech: Consultancy; Millenium: Consultancy; Pharmacyclics: Consultancy; Spectrum: Consultancy. Zelenetz:GSK: Consultancy; Celgene: Consultancy; Cephalon: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Sanofi-Aventis : Consultancy; Genentech: Research Funding; GSK: Research Funding; Roche: Research Funding; Cancer Genetics: Scientific Advisor Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal