Abstract
Primary Mediastinal large B-cell lymphoma (PMBL) is a rare form of Non Hodgkin Lymphoma (NHL) representing 2% of mature B-cell non-Hodgkin lymphoma in patients less than 18 years of age (Lones/Cairo et al., JCO, 2000; Burkhardt et al., BJH, 2005). PMBL has similar histo-pathological features along the biologic spectrum between Diffuse Large B-Cell Lymphoma (DLBCL) and classical HL (cHL) (Abramson et al., Blood, 2005). Gene expression studies suggested that the molecular signature of PMBL had a striking resemblance to the expression profile of cHL (Rosenwald et al., J Exp Med, 2003). We have recently reported that a significant decrease in EFS among children and adolescent PMBL patients compared with other stage III non-PMBL pediatric DLBCL patients following FAB/LMB 96 therapy, suggesting that children and adolescent PMBL may be an inherently different B-NHL (Gerrard/Cairo et al., Blood, 2013). Since over 95% of PMBL express CD20, targeting the CD20 receptor with a CD20 antibody is of high clinical interest. Obinutuzumab (GA101) is novel glycoengineered anti-CD20 targeted monoclonal antibody recognizing a unique CD20 type II epitope and it has been demonstrated to have greater efficacy in reducing tumor size, inducing remission and improving survival in other B-NHL xenograft models (Mössner et al., Blood, 2010).
We hypothesize that obinutuzumab may be a future potential targeted agent for the treatment of PMBL, and therefore, we investigated whether obinutuzumab significantly induces programmed cell death and reduces cell proliferation in PMBL.
CD20+ PMBL, Karpas-1106P cells were obtained from the DSMZ and obinutuzumab was provided by C. Klein, Roche Glycart AG. Total RNA was prepared using Trizol reagent (Invitrogen) from Karpas-1106P cells and cDNA was synthesized using cDNA Synthesis Kit (Quantas). qRT-PCR was performed using CFX96 Real-time system with SsoFast Supermix (Bio-rad). Relative quantification (ddCt) of mRNA expression of CD20 genes was determined by normalizing to GAPDH. Antibodies for western blotting were obtained from Cell signaling Tech. and Santa Cruz Bio., respectively. MTS (Promega) and Caspase 3/7 assay (Promega) were employed for cell proliferation and apoptosis assay with treatment of obinutuzumab (1, 10, 20 and 100ug/ml) at every 24hours. Cell death was assayed by FACS (MoFlo XDP Sorter, Beckman Coulter) of FITC-Annexin V and propidium iodide (PI)–stained cells (BD Biosciences) according to the manufacturer's instruction. Statistical significance was determined by one-tailed Student t-test.
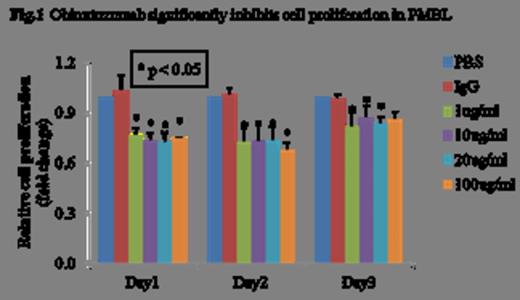
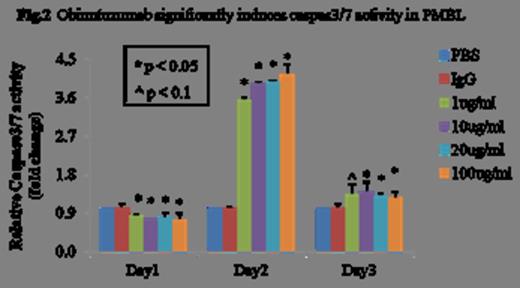
We observed a significantly higher expression of CD20 mRNA and protein in Karpas-1106P (>5.5-fold, p<0.01 and >2.5-fold, p<0.01) compared to the positive control, CD20+ Raji cells. There was a significant decrease of cell proliferation in obinutuzumab treated Karpas-1106P vs control cells at 24, 48 and 72 hours regardless of the dose of obinutuzumab with 23% reduction (p<0.05), 22% reduction (p<0.05), 24% reduction (p<0.05) and 24% reduction (p<0.05) with 1, 10, 20 and 100ug/ml of obinutuzumab treatment, respectively (Figure 1). A significant increase of Caspase 3/7 activity was observed at day 2 with >3.6-fold (p<0.03), >3.9-fold (p<0.03), >4.0-fold (p<0.02) and >4.2-fold increases (p<0.03) at escalating doses of obinutuzumab treatment, respectively compared to control (Figure 2). Treatment of PMBL cells with obinutuzumab (10 ug/ml) for 48 hours, CD20 expression were significantly decreased 26% (p<0.05) and 10% (p<0.05) in mRNA expression and protein levels, respectively. There was a significant increase of cell death in obinutuzumab treated Karpas-1106P compared to control (PBS) (37.8 vs 1.2%, p<0.02) using Annexin V-binding assay. Additionally, we assessed the expression of the pro-apoptotic gene, Bcl-xL and anti-apoptotic gene, Bax at both the mRNA and protein level. There were significant down-regulation of Bcl-xL (10% reduction, p<0.04) at both of the mRNA and protein levels, and a significant decrease in mRNA expression of Bax (30% reduction, p<0.02).
Obinutuzumab induced a significant increase in PMBL cell death and may be a future potential targeted agent for the treatment of PMBL lymphomas. Future studies will focus on the in vivo effects of obinutuzumab in a NOD/SCID PMBL xenograft mouse model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal