Abstract
The outcome of patients with CML has improved significantly since the introduction of imatinib (O’Brien et al 2003). In the landmark IRIS trial, the rate of complete cytogenetic response (CCyR) was 69% by 12 months and 87% by 60 months and 7% of patients progressed to accelerated or blast crisis (Druker et al 2006). Multiple studies show that bcr-abl transcript level at 3 months can predict outcome and can be used to guide therapeutic strategies (Marin et al 2012). Additionally, patients achieving CCyR within 2 years on imatinib were projected to have a normal life expectancy (Gambacorti-Passerini et al 2011). Surprisingly, a survey of the CML World Registry involving over 1800 patients revealed that only 10% and 15% of patients had cytogenetic and molecular evaluation at 3 months, respectively. At our institution, we have a dedicated, multidisciplinary CML clinic with RT-PCR assay in house. We sought to determine how the outcome of our CP-CML patients, followed every 3 months and according to the ELN (European Leukemia Network) guidelines compares to clinical trial results.
Patients with CP-CML treated with imatinib first line between January 2004 and December 2012 were included in this retrospective chart review. Diagnosis was based on bone marrow aspirate with blast count and cytogenetic and/or FISH, and bcr/abl transcript RT-PCR using the IS method. Patients presenting in AP or BP, or those having previous lines of therapy except hydroxyurea or anagrelide were excluded. Baseline demographic, medical history, Sokal score at presentation and monitoring frequency for hematologic, cytogenetic and molecular responses were extracted. Definitions of cytogenetic and molecular responses were in accordance with the ELN guidelines. Cumulative rates of cytogenetic and molecular responses were estimated using the Kaplan–Meier method. Data for patients not achieving an adequate response were censored at the last follow up visit. Differences between groups were calculated by log rank test. The study received IRB approval at our institution.
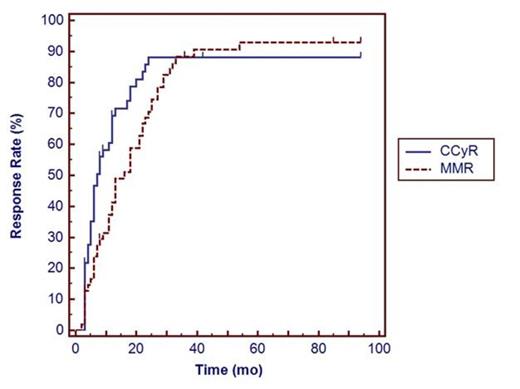
55 eligible patients were identified. Median age was 53 years (17-92) and 33 (60%) were male. Distribution of Sokal score was low in 28 (50.9%), intermediate in 22 (40%) and high in 5 (9%). Median follow up duration was 53 months (range 3-106). Seven patients (12.7%) required switching to second line TKI, six for inadequate response, one for intolerance and one patient required switch to third line TKI for inadequate response. Molecular testing at 3 and 18 months post start of imatinib was performed in 48 pts (87.3%) and 53 (96.4%), respectively. At 12 months, 49 (89.1%) had cytogenetic evaluation. Estimated rates of CCyR at 6 months, 12 months, 18 months and 24 months were 46.6%, 69.2%, 78.7% and 88.2%, respectively. Estimated rates of MMR at 6 months, 12 months, 18 months and 24 months were 23.9%, 41.2%, 58.8% and 70.6%, respectively. When stratified by Sokal score, rate of CCyR at 12 months was 82.1%, 72.7% and 60% (p 0.8840) for low, intermediate and high scores, respectively.
CP-CML patients followed in a dedicated CML clinic receive more rigorous follow-up and monitoring and achieve response rates similar to that of clinical trials. Additionally, in this small cohort of patients, discontinuation rate of imatinib was substantially lower than previously reported.
Kaplan–Meier estimates of the cumulative CCyR (solid line) and MMR (dotted line) response rates
Kaplan–Meier estimates of the cumulative CCyR (solid line) and MMR (dotted line) response rates
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal