Abstract
Chronic myeloid leukemia (CML) patients with imatinib failure are usually treated with second generation tyrosine kinase inhibitors (TKI). In case of dasatinib or nilotinib intolerance or resistance patients with HLA matched donor are submitted to allogeneic hematopoietic stem cell transplantation (HSCT) or switch treatment to a different TKI.
to evaluate complete hematological response (CHR), cytogenetic and molecular responses, overall, progression free and event free survival (OS, FPS and EFS) in CML patients in third line therapy that previously used 2 TKI. BCR-ABL mutations was also evaluated in this population
between July 2008 and August 2012 26 CML patients were evaluated: 10 patients (pts) treated with dasatinib (38,5%) and 16 with nilotinib (61,5%) in third line therapy. OS, PFS and EFS were calculated with Kaplan-Meier method and Log-Rank was used for comparison, from the date of the third TKI start. The events for PFS were progression to accelerated phase (AP) or blast crisis (BC) and death. For EFS, were considered loss of CHR, loss of complete cytogenetic response (CCR), TKI discontinuation for failure or intolerance, progression or death. Patients were censored in HSCT date or TKI discontinuation for cytogenetic and molecular responses evaluation, but not for OS. Mutational analysis was done with Sanger sequencing.
13 male (50%) and 13 female patients with median age 54 years (22-75) and median follow-up of 32 months were analyzed (1-59). Previous response to imatinib: only one patient has achieved CCR. Status before third TKI start: 19 (73%) less than partial cytogenetic response (PCyR); 2 (7,7%) PCyR; 4 (15,5%) CCR;and 1 (3,8%) clonal evolution. 8/18 (42%) patients presented the following mutations: F317L (2), E255V (1), Y253H (1), M351T (1), M244V (1) e F359V (2). The median time of the third TKI treatment was 171 days (15-1140). Responses to third line TKI: CHR 17/19 (89%) in CP; 3/3 AF; 0/4 BC; CCR: 3/19 in CP (2 pts at 3 months and one in the 12º month); 1/3 AP; 0/4 BC; MMR: 4/19 in CP; 1/3 AP; 0/4 in BC. Nine lost CHR (45%) (8 in CP and one in AP).
At 3 months, 1/21 pts (4.5%) achieved MMR; 2/13 (15%) at 6 months, while at 12 months 1/11 (9%) had MMR. At 3 months 11/21 had a BCR-ABL/ABL(IS) >10% and at 6 months 10/13 >1%. Three patients progressed to advanced phases (11,5%) ; 6 (23%) died (4 due to LMC, 1 acute myocardial infarction and 1 from graft vs host disease after HSCT; one lost follow-up. Third line treatment was discontinued in 12 (46%) pts: 6 for resistance, 2 death; 2 intolerance and resistance; 1 intolerance and one loss of follow-up.
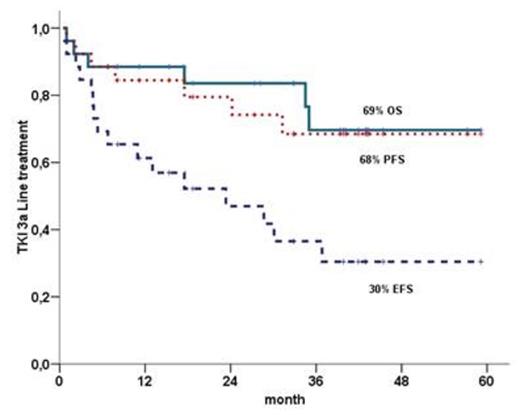
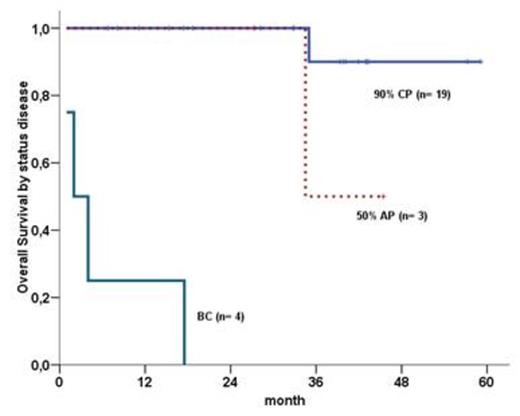
OS, PFS and EFS were 69%, 68% e 30% respectively(figure 1). OS was inferior in AP and BC in comparison with CP (0% vs 50% vs 90% - p< 0,0001)(figure 2).
responses may be obtained with dasatinib or nilotinib after 2 TKI failure, but they are not durable in advanced phases. Besides the results, that is an alternative for disease stabilization until the identification of a suitable donor or for patients with no performance status for HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal