Abstract
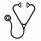
Treatment recommendations for childhood Immune Thrombocytopenia (ITP) in the United States have been a subject of much debate. In early 2011, the American Society of Hematology (ASH) updated their guidelines, recommending that children with ITP be managed with observation alone when there is mild or no bleeding symptoms regardless of platelet count, reserving hospitalization and pharmacologic treatment for those with significant bleeding. The objective of this study was to better understand the impact these recommendations have had on practice patterns at a large, urban, pediatric tertiary care hospital in the United States.
We examined data from patients aged 0-17 with newly diagnosed ITP seen in inpatient and outpatient locations at the Children's Hospital of Philadelphia (CHOP) between January 1, 2007 and December 31, 2012. A cohort was developed by querying the hospital data system for all patients treated 2007-2012 using the ICD-9 code for ITP (287.31). Chart review was used to exclude patients with alternative diagnosis for thrombocytopenia, diagnosis prior to the time of interest, and initial treatment elsewhere. We compared management strategies at diagnosis and retreatment rates in the first 6 months after the initial diagnosis. The primary outcome, management at diagnosis, was analyzed by logistic regression and chi square analysis.
502 unique patients were identified. After chart review, an evaluable cohort of 313 patients with newly diagnosed ITP remained. The most common reasons for elimination were an alternative diagnosis (n=41), diagnosis in a previous year (n=68), and initial treatment at another hospital (n=69). Of those with an alternative diagnosis, Evan's syndrome (n=8) and ALPS (n=5) were the most common reasons for elimination. New diagnoses per year ranged from 34-51 in 2007-2009 and 60-63 in 2010-2012. The mean platelet count and age at diagnosis were 16.9 x10^9/l and 6.22 years old, respectively. Distribution was skewed toward lower platelet count and younger age. Overall, 19.5% of patients were noted to have bleeding symptoms beyond bruising or petechiae. The most common bleeding symptoms at presentation were epistaxis (n=25), wet purpura (n=20), gastrointestinal bleeding (n=5), hematuria (n=4), and menstrual bleeding (n=4). Intracranial hemorrhage was rare (n=2, 0.6%). Overall, 55% of patients were managed with pharmacologic treatment at diagnosis. Of those treated, 98% were treated with IVIG. Additionally, the proportion of patients observed at diagnosis rose significantly during this time period from 34% of patients in 2007-2010 to 49.2% in 2011 (p<.02) and 72% in 2012 (p<0.001). Via logistic regression, younger age, lower platelet count, and year of diagnosis were significantly associated with increased odds of pharmacologic treatment. During 2010-2012, 21% of patients were also treated within 0.25-6 months after diagnosis, with no significant difference by year or initial management (p>0.5). The majority treated after diagnosis were due to “low platelet counts” (n=14), bruising/wet purpura (n=6), or parental anxiety/activity level (n=3). One child, initially treated with IVIG, presented with ICH secondary to trauma 1.5 months after diagnosis.
Conclusions
Over time, a significantly increased proportion of patients were observed at diagnosis. Though we cannot prove causation, this change demonstrates a strong association between timing of ASH recommendations and increase in observation rates. As we increased the proportion of children being observed at diagnosis we did not see an increase in the proportion of children receiving treatment or those with significant bleeding symptoms in the following 6 months. Additionally, we found that 37% of patients originally identified via ICD-9 code did not meet our criteria for evaluation of ‘newly diagnosed ITP'. As there is a greater shift to observing patients, along with the development of outpatient infusion services, inpatient databases could become skewed in regard to ITP. Because of this, caution should be utilized in using ICD-9 code alone to identify new diagnoses, especially when chart review is not accessible. Future studies will be able to identify the changing national trend in admission and management and whether adoption of ASH guidelines is widespread.
Lambert:Amgen: Research Funding; Nestle: Consultancy; GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal