In this issue of Blood, Wang et al identify an important role for platelet-derived extracellular ERp57, a thiol isomerase enzyme, in platelet integrin regulation and recruitment into a growing thrombus.1
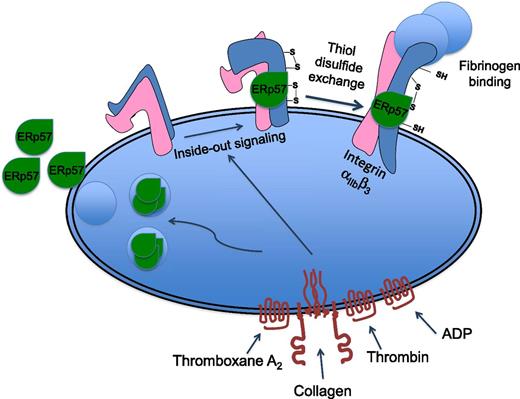
Platelet activation results in the relocation of the thiol isomerase enzyme ERp57 to the platelet surface where it binds to the β3 integrin subunit. The absence of ERp57 protein, or inhibition of its activity, results in diminished platelet activation, aggregation, and recruitment into a growing thrombus. Because integrin αIIbβ3 activation, which is necessary for fibrinogen binding, is associated with disulfide rearrangement, this suggests ERp57 may contribute to its activation or stabilization in a conformation that is able to bind to fibrinogen.
Platelet activation results in the relocation of the thiol isomerase enzyme ERp57 to the platelet surface where it binds to the β3 integrin subunit. The absence of ERp57 protein, or inhibition of its activity, results in diminished platelet activation, aggregation, and recruitment into a growing thrombus. Because integrin αIIbβ3 activation, which is necessary for fibrinogen binding, is associated with disulfide rearrangement, this suggests ERp57 may contribute to its activation or stabilization in a conformation that is able to bind to fibrinogen.
Platelets might seem like simple cells—but are they? They lack a nucleus and therefore do not have to worry about gene transcription, and as a consequence, they have only a limited life expectancy in the circulation, before being cleared and replaced by new platelets. Over recent years, however, the molecular processes that control the functions of platelets in hemostasis and thrombosis have begun to emerge and have revealed platelets to be perhaps more complex that may have been anticipated.
Curiously, platelets contain many proteins that one may not expect them to need, at least not if they use these proteins in a conventional manner. It seems, however, that platelets are creative and use some proteins in unexpected ways. A prime example of this is a family of enzymes known as thiol isomerases.2 These proteins, the best characterized of which is protein disulfide isomerase (PDI), normally function within the endoplasmic reticulum of cells, where they perform an important role in ensuring the correct folding of proteins before they are secreted to the cell surface or beyond. They function to catalyze the reversible oxidation of thiols to disulfide bonds and the rearrangement of disulfide bonds. Together with other chaperone proteins, their location is normally restricted to a cell’s secretory machinery where they enable proteins to achieve the correct conformation for their function.
Platelets contain PDI and a number of other thiol isomerases such as ERp5 and ERp57, and curiously, they find their way to the outside of the cell, whereupon they bind to the platelet surface.2-4 Selective inhibition of enzymatic function results in diminished platelet activation and thrombus formation.3-6 Therefore, although platelets do not engage in protein synthesis (other than low-level translation of remnant megakaryocyte mRNA), this suggests that protein structure is processed at the platelet surface where numerous disulfide-bonded proteins reside. Notably, these include integrin αIIbβ3, the receptor for fibrinogen that supports platelet thrombus formation and to which extracellular ERp5 and PDI have been shown to bind.
The culmination of platelet activation is the conversion of this receptor from a low affinity state to a high affinity (fibrinogen-binding) state, and this process is associated with disulfide rearrangement. It is possible, therefore, that integrin αIIbβ3 may represent a key thiol isomerase substrate at the cell surface, although other possibilities including integrin α2β1 have been suggested. PDI has been shown to be released by activated or damaged endothelial cells, suggesting other potential sources of extracellular thiol isomerases at sites of vascular injury.7 It is also implicated in the “de-encryption” of tissue factor expressed at sites of injury, converting it into a form that activates coagulation.8
A number of key questions therefore arise. What are the roles and mechanisms of action of different thiol isomerases in hemostasis and thrombosis, and specifically are platelet thiol isomerases important for these functions? In this issue, Wang et al report that platelet ERp57 plays a major role in the activation of integrin αIIbβ3 and platelet accumulation into a growing thrombus.1
Transgenic mice were created in which the ERp57 gene is deleted in megakaryocytes and platelets. Although platelet numbers and receptor expression appeared to be normal in these mice, tail bleeding times were extended, and thrombus formation was delayed. Platelet recruitment into experimentally induced thrombi, formed in mesenteric arterioles, was also delayed. It appeared therefore that other thiol isomerases are unable to compensate for the lack of platelet ERp57 in the early phases of thrombus formation, suggesting distinct roles for different family members. Platelet recruitment to thrombi recovered over time, however, indicating that some compensation may occur, potentially by ERp57 from other cellular sources.
Deficiency in platelet ERp57 resulted in decreased activation of integrin αIIbβ3 and platelet aggregation, consistent with the effects of ERp57-inhibiting antibodies,5,6 and this was recovered by addition of recombinant (extracellular) ERp57. The addition of exogenous ERp57 also potentiated the activation of thrombin-activated human platelets, and through analysis of ERp57 mutant proteins, this activity was mapped to its second active site.
The possibility that ERp57 binds to β3 integrins at the platelet surface was explored using β3-deficient platelets. The binding of fluorescently labeled ERp57 to thrombin-activated platelets was reduced substantially in the absence of β3. Interestingly, residual activation-dependent ERp57 binding to β3-deficient platelets was observed, indicating that other binding sites exist for this protein on the platelet surface.
It appears therefore, that platelet ERp57 is important for the activation of platelets in vivo, their recruitment into a developing thrombus, and thereby the control of hemostasis and thrombosis. Similar observations have been recently reported following the deletion of PDI expression in mouse platelets.9 The treatment of PDI-deficient platelets with an anti-ERp57 enzyme blocking antibody was shown to further diminish integrin αIIbβ3 activation and platelet aggregation, further supporting the hypothesis that PDI and ERp57 have distinct roles in the control of platelet function.9
A future priority will be to determine the specific substrates (shared or otherwise) of the range of thiol isomerases that control platelet function at the cell surface and to establish whether these roles also extend to the regulation of tissue factor function. The development of mice deficient in specific family members and the availability of selective inhibitors will provide important tools for this. The observed contributions of platelet ERp57 and PDI (directly or indirectly) to the modulation of integrin αIIbβ3 affinity suggest that the selective inhibition of extracellular thiol isomerases may enable new antithrombotic drug development. Indeed, screens to identify thiol isomerase inhibitors have led to the proposal that they may constitute a new class of antithrombotic agents.10 A challenge will be to develop inhibitors that are unable to gain access to intracellular thiol isomerases, given their vital and ubiquitous roles in protein folding.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal