In this issue of Blood, Doyle et al provide evidence that knockout of the genes encoding the two most abundant eosinophil secondary granule proteins disrupts the normal differentiation of eosinophils from progenitors in the bone marrow, providing a novel strain of mice with a highly specific deficiency in eosinophilopoiesis and, therefore, eosinophils. This strain is likely to be used by investigators to elaborate the normal vs pathogenic roles of eosinophils in health and disease.1
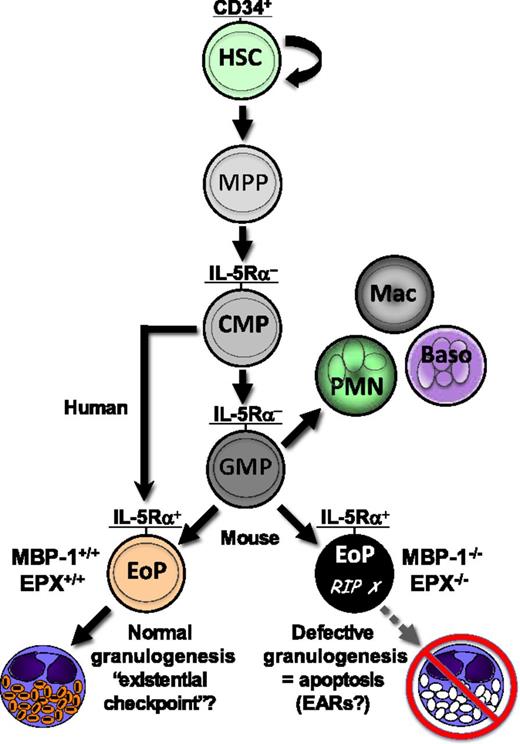
Schematic of normal hematopoietic development of the eosinophil granulocyte and impact of knocking out genes encoding the two most abundant eosinophil secondary granule proteins. EoPs, defined by expression of a high-affinity receptor for IL-5 (IL-5Rα), develop from common myeloid progenitors (CMPs) in humans and from GMPs in the mouse. Double deletion (knockout) of the murine genes encoding eosinophil granule MBP-1 and EPX (MBP-1−/−/EPX−/−) leads to impaired development of eosinophil secondary granules (defective granulogenesis) in EoPs, inducing programmed cell death (apoptosis) and a consequent profound eosinophil deficiency in these mice. The results of the study by Doyle et al1 suggest that normal granulogenesis may be a novel checkpoint for successful eosinophil differentiation, such that defective granulogenesis leads to impaired survival (apoptosis) of developing eosinophils, or that loss of expression of MBP-1 and EPX disrupts lineage-instructive gene regulatory mechanisms affecting continued EoP self-renewal and/or survival. Baso, basophil; EARs, eosinophil-associated ribonucleases; HSC, hematopoietic stem cell; Mac, monocyte/macrophage; MPP, multi-potential progenitor; PMN, polymorphonuclear neutrophil; RIP, rest in peace.
Schematic of normal hematopoietic development of the eosinophil granulocyte and impact of knocking out genes encoding the two most abundant eosinophil secondary granule proteins. EoPs, defined by expression of a high-affinity receptor for IL-5 (IL-5Rα), develop from common myeloid progenitors (CMPs) in humans and from GMPs in the mouse. Double deletion (knockout) of the murine genes encoding eosinophil granule MBP-1 and EPX (MBP-1−/−/EPX−/−) leads to impaired development of eosinophil secondary granules (defective granulogenesis) in EoPs, inducing programmed cell death (apoptosis) and a consequent profound eosinophil deficiency in these mice. The results of the study by Doyle et al1 suggest that normal granulogenesis may be a novel checkpoint for successful eosinophil differentiation, such that defective granulogenesis leads to impaired survival (apoptosis) of developing eosinophils, or that loss of expression of MBP-1 and EPX disrupts lineage-instructive gene regulatory mechanisms affecting continued EoP self-renewal and/or survival. Baso, basophil; EARs, eosinophil-associated ribonucleases; HSC, hematopoietic stem cell; Mac, monocyte/macrophage; MPP, multi-potential progenitor; PMN, polymorphonuclear neutrophil; RIP, rest in peace.
In the more than 100 years following Paul Ehrlich’s 1879 identification of the eosinophil, the compendium of diseases and idiopathic syndromes characterized by blood or tissue eosinophilia grew by leaps and bounds, while our understanding of the functional roles of the eosinophil in innate immunity and host defense, allergic responses, tissue injury/repair, and remodeling/fibrosis lagged far behind, being addressed in only the past 30 or so years.2 Initial studies characterized the unique biologic characteristics of blood and tissue eosinophils, their preformed secondary granule proteins, and inducible lipid, oxidative, and cytokine products, focusing on the eosinophil’s proinflammatory and cytotoxic potential in the pathogenesis of allergic, parasitic, and a variety of idiopathic eosinophil-associated syndromes. Recognition of the eosinophil as an effector cell in asthma pathogenesis fueled an initial surge in eosinophil interest, while an “epidemic” of eosinophil myalgia syndrome from ingestion of tainted l-tryptophan, and more recent identification of the food-allergic disease eosinophilic esophagitis,3 markedly increased clinical interest and public awareness of this granulocyte.
The current paradigm—that eosinophils subserve proinflammatory tissue-damaging and tissue-remodeling roles in eosinophil-associated diseases—is supported by a growing number of definitive mouse model and human studies. A pivotal role for the eosinophil in the development of tissue remodeling and fibrosis, through elaboration of remodeling and fibrogenic factors (eg, transforming growth factor beta), is widely accepted.4 Studies using two strains of eosinophil-deficient mice (PHIL and ΔdblGATA)5,6 strongly support the concept that eosinophils contribute to the pathology of airway remodeling in asthma and are required for T-cell polarization for development of Th2 responses in the lung in response to allergen challenge.7 Clinical trials using anti–interleukin-5 (IL-5) antibody to ablate eosinophils in bone marrow, blood, and tissues of patients with eosinophilic, but not neutrophilic, asthma showed efficacy in reversing aspects of eosinophil-mediated tissue damage, remodeling, fibrosis, and airway dysfunction8 and pathologies associated with the hypereosinophilic syndrome.9 Thus, the availability of two strains of eosinophil-deficient mice, PHIL5 and ΔdblGATA,6 has been integral to understanding the contributions of eosinophils to disease pathogenesis and normal tissue homeostasis.
Expression of the major eosinophil granule cationic proteins, major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX), is the consequence of normal hematopoietic development of eosinophil lineage-committed progenitors (EoPs) leading to terminal differentiation of the mature eosinophil or, under conditions of increased expression of IL-5 from Th2 T cells and other cell sources, expansion of the EoP population, which then leads to blood and tissue eosinophilia. The study by Doyle et al1 reports the paradoxical finding that concurrent expression of MBP-1 and EPX appears to be required for and reinforces the development of the eosinophil lineage, or conversely, that the absence of expression (double gene knockout, MBP-1−/−/EPX−/−) leads to a failure of commitment of granulocyte-monocyte progenitors (GMPs) to the eosinophil lineage, loss of proliferative potential of EoPs, or aborted differentiation of committed EoPs. The findings appear counterintuitive, since it is not immediately clear whether loss of terminally differentiated eosinophils is due to the absence of required genetic loci deleted along with coding regions in the double knockout of the MBP-1 and EPX genes or due to the absence of expression of the MBP-1 and EPX proteins and consequent negative effects on EoP development in terms of defective granulogenesis (see figure). Importantly, the authors effectively eliminate most of these potential explanations, providing evidence instead that EoPs in these double MBP-1−/−/EPX−/− knockout mice selectively undergo apoptosis, with no observable effects on the common myeloid progenitors or GMP populations (see figure). Although the authors do not provide mechanistic evidence for induction of EoP apoptosis in the MBP-1−/−/EPX−/− null mice, they do provide speculative mechanistic insights that eliminate a number of possible explanations and suggest others for this effect: (1) granule protein gene expression and/or granulogenesis is an existential checkpoint for survival of EoPs, and/or (2) loss of concurrent expression of MBP-1 and EPX in EoPs disrupts lineage-instructive gene regulatory mechanisms that contribute to continued self-renewal and/or EoP survival (see figure). However, invoking Occam’s razor, I suggest that one of the most straightforward and simple explanations for the observed induction of EoP apoptosis in the face of dysfunctional granulogenesis in the MBP-1−/−/EPX−/− null mice may be aberrant intracellular release of a toxicant, the mouse eosinophil-associated ribonucleases (EARs), which are capable of rapidly degrading intracelluar RNA, thus leading to the observed cell-autonomous defect. Of note, defective granulogenesis, with deficient expression of the secondary granule protein MBP-1 and two human EARs (eosinophil-derived neurotoxin [EDN or RNase 2] and eosinophil cationic protein [ECP or RNase 3]) has been described in patients with specific granule deficiency (SGD)10 and has been ascribed to a frameshift mutation in the gene encoding the transcription factor CCAAT-enhancer-binding protein epsilon, which is required for granulocyte (both neutrophil and eosinophil) progenitor terminal differentiation. Although SGD impairs differentiation to functionally active eosinophils, it does not appear to limit eosinophilopoiesis in the absence of the two human EARs, which supports their potential role in inducing EoP apoptosis in the MBP-1−/−/EPX−/− knockout mice.
In summary, the study by Doyle et al characterizes a novel eosinophil-deficient mouse strain that lacks many of the problems and criticisms associated with the currently available eosinophil-deficient strains (PHIL and ΔdblGATA), since the defect is entirely eosinophil specific (affects only EoPs in a cell-autonomous manner) and does not involve the lineage-specific expression of a toxin (diptheria toxin A chain) in developing EoPs (in PHIL) or have other collateral defects (eg, on red cell development in ΔdblGATA) due to dysregulation of GATA-1 transcriptional autoregulation. Although the specific mechanisms underlying the eosinophil deficiency in the MBP-1−/−/EPX−/− knockout mice remain to be determined, this novel strain is certain to be adopted by investigators for mouse model studies of the normal vs pathogenic roles of eosinophils in health and disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.