Key Points
Interaction of HTLV-1 Tax with USP10 reduces arsenic-induced stress granule formation and enhances ROS production.
USP10 controls sensitivities of leukemia cell lines to arsenic-induced apoptosis.
Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL), and the viral oncoprotein Tax plays key roles in the immortalization of human T cells, lifelong persistent infection, and leukemogenesis. We herein identify the ubiquitin-specific protease 10 (USP10) as a Tax-interactor in HTLV-1–infected T cells. USP10 is an antistress factor against various environmental stresses, including viral infections and oxidative stress. On exposure to arsenic, an oxidative stress inducer, USP10 is recruited into stress granules (SGs), and USP10-containing SGs reduce reactive oxygen species (ROS) production and inhibit ROS-dependent apoptosis. We found that interaction of Tax with USP10 inhibits arsenic-induced SG formation, stimulates ROS production, and augments ROS-dependent apoptosis in HTLV-1–infected T cells. These findings suggest that USP10 is a host factor that inhibits stress-induced ROS production and apoptosis in HTLV-1–infected T cells; however, its activities are attenuated by Tax. A clinical study showed that combination therapy containing arsenic is effective against some forms of ATL. Therefore, these findings may be relevant to chemotherapy against ATL.
Introduction
Environmental stresses, such as hypoxia, heat shock, UV irradiation, and arsenite, induce several alterations in cells, such as DNA damage and accumulation of misfolded proteins, the short- and long-term consequences of which include survival of cells with aberrant DNA and protein alterations, inflammation, aging, and carcinogenesis. On the other hand, cells activate several defense mechanisms to protect against these outcomes. For instance, cells transiently induce the formation of cytoplasmic RNA granules designated as stress granules (SGs).1,2 SGs have several antistress functions. The stresses induce translational arrest, leading to polysome disassembly, and SGs transiently store translationally inactive messenger RNAs released from polysomes and promote the translation of stress-repairing proteins, such as heat shock proteins. In addition, SGs inhibit reactive oxygen species (ROS) production and ROS-dependent apoptosis,3 and in the meantime, cells repair altered DNA and remove misfolded proteins. Therefore, SGs play critical roles in protecting against stress-induced alterations in cells.
Viral infections have also been shown to induce the formation of SGs in host cells, although some viruses encode proteins that inhibit the formation of SGs.4 For instance, the poliovirus-encoded protease 3C protein prevents SG formation via protease-dependent cleavage of Ras-GTPase–activating protein-binding protein 1 (G3BP1),5 which is essential for SG assembly.6 In addition, the NS1 encoded by the influenza A virus inhibits SG formation by preventing the activation of protein kinase that initiates a phosphorylation cascade leading to SG formation after viral infection.7 On the basis of these observations, SGs are proposed to be involved in innate immunity against viral infections, and the inhibition of SG formation stimulates viral replication and production and augments inflammation and tissue damage.8
Human T-cell leukemia virus type 1 (HTLV-1) is an etiologic agent of adult T-cell leukemia (ATL).9 Accumulation of genetic and epigenetic aberrations in HTLV-1–infected cells is a crucial step for ATL development, as only 5% of HTLV-1–infected individuals go on to have ATL at an average of 40 years after infection. Indeed, ATL cells have multiple DNA alterations, such as mutations of cellular oncogenes and tumor suppressor genes. HTLV-1 encodes the Tax oncoprotein, which plays crucial roles in the immortalization of virus-infected T cells, accumulation of gene mutations in infected cells, and leukemogenesis.9,10 To perform such pleiotropic functions, Tax exhibits a variety of activities, including transcriptional activation of a number of cellular genes via the actions of transcription factors nuclear factor κB (NF-κB), cAMP response element binding/activating transcription factor (CREB/ATF), and AP-1 and inactivation of the tumor suppressor gene p53.11-13 Notably, Tax also inhibits SG formation14 ; however, its significance in HTLV-1 pathogenicity remains to be clarified.
Ubiquitin-specific protease 10 (USP10) is a component of SGs that plays critical roles in several SG-mediated activities. USP10 is not essential for SG formation; however, its knockout in mouse embryonic fibroblasts (MEFs) reduces SG formation, augments ROS production, and enhances ROS-dependent apoptosis.3 In our current study, we identified USP10 as a novel binding partner of Tax protein. The activities of Tax mutants and knockdown of USP10 indicated that Tax, by interacting with USP10, inhibits SG formation and stimulates ROS production. Accumulating evidence indicates that aberrant ROS production is a factor promoting gene mutations that cause malignancy.15 Therefore, the present findings indicate that USP10 is a host factor that inhibits ROS-induced aberration of HTLV-1–infected T cells, including genetic mutations. Notably, a previous clinical study showed that combination therapy containing arsenic trioxide, an anhydrous form of arsenite, exhibits promising effects against some forms of ATL.16 Our results revealed that knockdown of USP10 augments arsenite-induced ROS-dependent apoptosis in HTLV-1–infected T cells. Therefore, USP10 controls the sensitivity of ATL cells to arsenic and is hence a novel target for chemotherapy against ATL.
Methods
Reagents and antibodies
The following reagents were purchased from the indicated companies: sodium arsenite (Wako Pure Chemical Industries), arsenic trioxide (Sigma-Aldrich), puromycin (Calbiochem) and N-acetylcysteine (NAC; Sigma-Aldrich). Arsenic trioxide was first dissolved in 1.0 N of NaOH and was then diluted with phosphate-buffered saline (PBS). The following antibodies were used in this study at the indicated dilutions: anti-USP10 (1:2000; Bethyl Laboratories), anti-HA (1:2000; Cell Signaling Technology), anti-G3BP1 (1:2000; BD Transduction Laboratories), anti-poly(A)-binding protein 1 (PABP1) (1:1000; Santa Cruz, 1:500; Abcam), anti-Tax (Taxy-7)17 (1:1000), and anti-α-tubulin (1:1000; Oncogene).
Coimmunoprecipitation and western blot analysis
The coimmunoprecipitation and western blot analysis were performed as described previously.3
Immunofluorescence analysis
An immunofluorescence analysis was performed as described previously.3 The images were analyzed with a fluorescence microscope (BZ-8000; KEYENCE). More than 300 cells in 3 random fields were analyzed using staining with SG markers, G3BP1 and USP10. The SG (%) was calculated as the ratio of SG-positive cells relative to the total number of cells.
Detection of ROS
Cellular ROS level was measured as described previously.3 The 5-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) fluorescence in each cell was quantified using a fluorescent analysis software package (BZ-II analyzer; KEYENCE). More than 400 cells in 4 random fields were analyzed, and the data were presented as the mean fluorescence intensity (DCFDA-F).
Quantitative determination of apoptosis
The level of apoptosis was measured according to 2 methods. First, the cells were stained with propidium iodide, and the sub-G1 DNA content was measured using flow cytometry. Second, cells cultured on coverslips in 6-well plates were fixed with 4% formaldehyde in PBS and were permeabilized with 0.1% Triton X-100 in PBS. The fixed cells were stained with Hoechst33258. After Hoechst33258 staining, the number of cells that exhibited condensed nuclei was counted using a fluorescence microscope. More than 300 cells in 3 random fields were analyzed per sample.
Statistical analysis
The data were analyzed using the unpaired Student t test and are presented as the mean ± standard deviation (SD). Information including “Cell lines and culture conditions,” “Plasmid constructs,” and the “Establishment of stable USP10-knockdown cell lines using lentiviral transduction” is provided in the supplemental Methods, available on the Blood Web site.
Results
Tax interacts with USP10
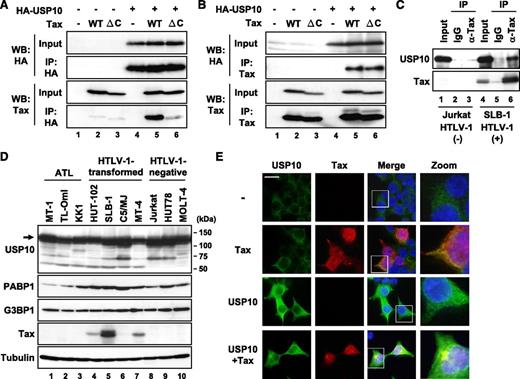
Cytotoxic T lymphoblast cell (CTLL)-2 is a mouse interleukin (IL)-2–dependent T-cell line. Tax transforms CTLL-2 cells from IL-2–dependent growth into IL-2–independent growth, and its activity is much higher than that of nonleukemogenic HTLV-2 Tax2.18,19 Studies of chimeric Tax proteins including Tax2 have revealed that the C-terminal PDZ domain-binding motif (PBM) in Tax, which is missing in Tax2, is a factor responsible for the high transforming activity of CTLL-2.18,19 To obtain information on how Tax PBM augments the Tax-induced transformation of CTLL-2, we isolated several Tax-binding proteins in CTLL-2 cell lysates using glutathione S-transferase fusion proteins of Tax vs TaxΔC with deletion of PBM (M.H. and M.F., unpublished observations). One such Tax-binding protein is USP10. To establish the interaction between Tax and USP10 in mammalian cells, plasmids encoding Tax as well as USP10 with a hemagglutinin (HA) epitope (HA-USP10) were transiently expressed in 293T cells, and the cell lysates were immunoprecipitated with anti-HA antibodies. The anti-HA antibody coprecipitated HA-USP10 with Tax (Figure 1A). Conversely, anti-Tax coprecipitated HA-USP10 with Tax (Figure 1B). Unexpectedly, USP10 also interacted with the Tax PBM mutant TaxΔC (Figure 1B), indicating that PBM is not essential for the interaction between Tax and USP10. In addition, Tax formed a complex with USP10 in HTLV-1–infected T cells (Figure 1C). A total of 7 HTLV-1–positive and 3 HTLV-1–negative T-cell lines constitutively expressed USP10 as well as 2 USP10-binding proteins, G3BP120 and PABP1,21 and the levels of these factors did not correlate with that of Tax (Figure 1D). An immunofluorescence analysis visualized endogenous and exogenous USP10 in the cytoplasm, where some proteins colocalized with Tax (Figures 1E and 6A; as represented by arrows). Taken together, these results indicate that Tax interacts with USP10 in HTLV-1–infected T cells, and these molecules partly colocalize in the cytoplasm.
Tax interacts with USP10. (A,B) 293T cells were transfected with a control plasmid (lanes 1, 4), Tax plasmid (lanes 2, 5), or TaxΔC plasmid (lanes 3, 6) together with (lanes 4-6) or without (lanes 1-3) an HA-USP10 plasmid. At 48 hours after transfection, the cell lysates were immunoprecipitated with anti-HA (A) or anti-Tax (B) antibodies, and the total lysates (input) and immunoprecipitates (IP) were characterized using a western blot analysis with anti-HA and anti-Tax antibodies. TaxΔC has a 4 amino acid deletion on the Tax C-terminus, the peptide of which is missing in the nonleukemogenic HTLV-2 Tax2, because the original goal was to isolate binding factors specific to Tax but not to Tax2. (C) The cell lysates prepared from HTLV-1–uninfected T cells (Jurkat; lanes 1-3) and HTLV-1–infected T cells (SLB-1; lanes 4-6) were immunoprecipitated with anti-Tax antibodies (lanes 3, 6) or control antibodies (lanes 2, 5). The input lysate and IP were characterized using a western blot analysis with anti-USP10 and anti-Tax antibodies. (D) Cell lysates were prepared from 7 HTLV-1–infected T-cell lines (lanes 1-7) and 3 HTLV-1–uninfected T-cell lines (lanes 8-10). The expressions of USP10, PABP1, G3BP1, Tax, and α-tubulin proteins were determined using a western blot analysis with the corresponding antibodies. (E) 293T cells were transfected with a Tax plasmid together with or without the HA-USP10 plasmid. The transfected cells were stained with anti-USP10 (green) and anti-Tax (red) antibodies. The nuclei were counterstained with Hoechst33258 (blue). The bar indicates 20 μm.
Tax interacts with USP10. (A,B) 293T cells were transfected with a control plasmid (lanes 1, 4), Tax plasmid (lanes 2, 5), or TaxΔC plasmid (lanes 3, 6) together with (lanes 4-6) or without (lanes 1-3) an HA-USP10 plasmid. At 48 hours after transfection, the cell lysates were immunoprecipitated with anti-HA (A) or anti-Tax (B) antibodies, and the total lysates (input) and immunoprecipitates (IP) were characterized using a western blot analysis with anti-HA and anti-Tax antibodies. TaxΔC has a 4 amino acid deletion on the Tax C-terminus, the peptide of which is missing in the nonleukemogenic HTLV-2 Tax2, because the original goal was to isolate binding factors specific to Tax but not to Tax2. (C) The cell lysates prepared from HTLV-1–uninfected T cells (Jurkat; lanes 1-3) and HTLV-1–infected T cells (SLB-1; lanes 4-6) were immunoprecipitated with anti-Tax antibodies (lanes 3, 6) or control antibodies (lanes 2, 5). The input lysate and IP were characterized using a western blot analysis with anti-USP10 and anti-Tax antibodies. (D) Cell lysates were prepared from 7 HTLV-1–infected T-cell lines (lanes 1-7) and 3 HTLV-1–uninfected T-cell lines (lanes 8-10). The expressions of USP10, PABP1, G3BP1, Tax, and α-tubulin proteins were determined using a western blot analysis with the corresponding antibodies. (E) 293T cells were transfected with a Tax plasmid together with or without the HA-USP10 plasmid. The transfected cells were stained with anti-USP10 (green) and anti-Tax (red) antibodies. The nuclei were counterstained with Hoechst33258 (blue). The bar indicates 20 μm.
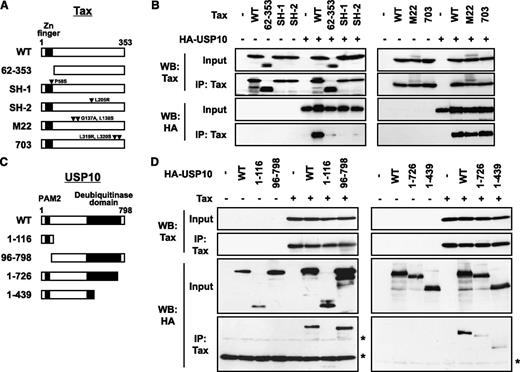
The domains of Tax and USP10 are required for the interaction. (A) A schematic representation of Tax and its mutants used in this study. (B) Cell lysates were prepared from 293T cells transfected with the HA-USP10 plasmid together with Tax mutant plasmids, and then were immunoprecipitated with anti-Tax. The total lysates (input) and immunoprecipitates (IP) were characterized using a western blot analysis with anti-Tax and anti-HA antibodies. (C) A schematic representation of USP10 and its mutants used in this study. PAM2 (PABP-interacting motif 2) mediates the interaction with PABP.21 (D) 293T cells were transfected with plasmids encoding HA-tagged USP10 (HA-WT) or its mutants (HA-1-116, HA-96-798, HA-1-726, or HA-1-439), as described in (C), together with Tax plasmids. Cell lysates prepared from 293T cells were then immunoprecipitated with anti-Tax antibodies. The input and immunoprecipitates were characterized using a western blot analysis with anti-Tax and anti-HA antibodies. The asterisks indicate nonspecific bands.
The domains of Tax and USP10 are required for the interaction. (A) A schematic representation of Tax and its mutants used in this study. (B) Cell lysates were prepared from 293T cells transfected with the HA-USP10 plasmid together with Tax mutant plasmids, and then were immunoprecipitated with anti-Tax. The total lysates (input) and immunoprecipitates (IP) were characterized using a western blot analysis with anti-Tax and anti-HA antibodies. (C) A schematic representation of USP10 and its mutants used in this study. PAM2 (PABP-interacting motif 2) mediates the interaction with PABP.21 (D) 293T cells were transfected with plasmids encoding HA-tagged USP10 (HA-WT) or its mutants (HA-1-116, HA-96-798, HA-1-726, or HA-1-439), as described in (C), together with Tax plasmids. Cell lysates prepared from 293T cells were then immunoprecipitated with anti-Tax antibodies. The input and immunoprecipitates were characterized using a western blot analysis with anti-Tax and anti-HA antibodies. The asterisks indicate nonspecific bands.
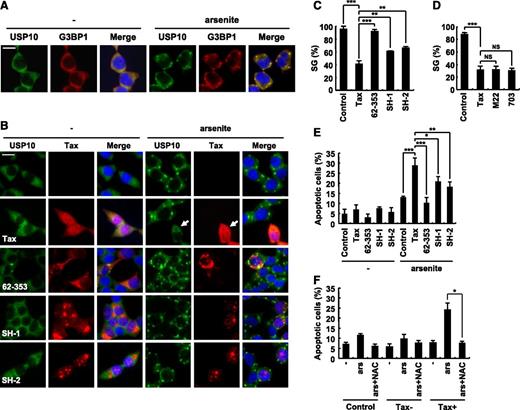
Tax inhibits SG formation and augments arsenite-induced apoptosis. (A) 293T cells were treated with 0.5 mM of sodium arsenite for 60 minutes and then were stained with anti-USP10 antibodies (green), anti-G3BP1 antibodies (red), and Hoechst33258 (blue). The bar indicates 20 μm. (B) 293T cells were transfected with the indicated plasmids (Tax, Tax62-353, TaxSH-1, or TaxSH-2). The transfected cells were treated with 0.5 mM of sodium arsenite for 60 minutes and then were stained with anti-USP10 antibodies (green), anti-Tax (red) antibodies, and Hoechst33258 (blue). The arrow indicates a cell that exhibits reduced SG formation. The bar indicates 20 μm. (C,D) The SG (%) in Tax-positive cells and control cells is presented. (E) The number of cells containing condensed nuclei among the Tax-positive cells was counted. (F) 293T cells were transfected with Tax plasmids and then were incubated with or without 5 mM of NAC, treated with 0.5 mM of sodium arsenite, and stained with anti-Tax antibodies and Hoechst33258. The numbers of cells containing condensed nuclei among the Tax-positive cells and the control cells were counted. In all experiments, the values denote mean ± SD; *P < .05; **P < .01; ***P < .001; NS indicates not statistically significant.
Tax inhibits SG formation and augments arsenite-induced apoptosis. (A) 293T cells were treated with 0.5 mM of sodium arsenite for 60 minutes and then were stained with anti-USP10 antibodies (green), anti-G3BP1 antibodies (red), and Hoechst33258 (blue). The bar indicates 20 μm. (B) 293T cells were transfected with the indicated plasmids (Tax, Tax62-353, TaxSH-1, or TaxSH-2). The transfected cells were treated with 0.5 mM of sodium arsenite for 60 minutes and then were stained with anti-USP10 antibodies (green), anti-Tax (red) antibodies, and Hoechst33258 (blue). The arrow indicates a cell that exhibits reduced SG formation. The bar indicates 20 μm. (C,D) The SG (%) in Tax-positive cells and control cells is presented. (E) The number of cells containing condensed nuclei among the Tax-positive cells was counted. (F) 293T cells were transfected with Tax plasmids and then were incubated with or without 5 mM of NAC, treated with 0.5 mM of sodium arsenite, and stained with anti-Tax antibodies and Hoechst33258. The numbers of cells containing condensed nuclei among the Tax-positive cells and the control cells were counted. In all experiments, the values denote mean ± SD; *P < .05; **P < .01; ***P < .001; NS indicates not statistically significant.
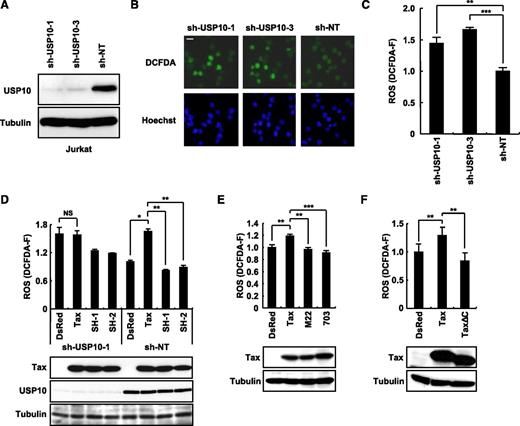
Tax stimulates ROS production in part through an interaction with USP10. (A) Jurkat cells were infected with lentiviruses encoding human USP10 shRNA (sh-USP10-1 or sh-USP10-3) or control nontargeting shRNA (sh-NT), and the cells were then cultured in the presence of puromycin. Cell lysates prepared from the selected cells were characterized using a western blot analysis with anti-USP10 and anti-α-tubulin antibodies. (B) USP10-knockdown Jurkat cells and control cells were stained with 5 μM of CM-H2DCFDA (green) and Hoechst33258 (blue) for 5 minutes at 37°C. Staining of the cells was visualized using a fluorescence microscope. The bar indicates 10 μm. (C) The ROS levels (DCFDA-F) in cells treated as in (B) were quantitatively measured using a cell imaging software program. (D-F) USP10-knockdown (sh-USP10-1) Jurkat cells and control (sh-NT) (D) or Jurkat cells (E,F) were infected with the indicated Tax lentiviruses. The infected cells were assessed for ROS production (DCFDA-F) using 5 μM of CM-H2DCFDA. Aliquots of the above-treated cells were subjected to a western blot analysis using anti-Tax, anti-USP10, and anti-α-tubulin antibodies. In all experiments, the values denote the mean ± SD; *P < .05; **P < .01; ***P < .001; NS indicates not statistically significant.
Tax stimulates ROS production in part through an interaction with USP10. (A) Jurkat cells were infected with lentiviruses encoding human USP10 shRNA (sh-USP10-1 or sh-USP10-3) or control nontargeting shRNA (sh-NT), and the cells were then cultured in the presence of puromycin. Cell lysates prepared from the selected cells were characterized using a western blot analysis with anti-USP10 and anti-α-tubulin antibodies. (B) USP10-knockdown Jurkat cells and control cells were stained with 5 μM of CM-H2DCFDA (green) and Hoechst33258 (blue) for 5 minutes at 37°C. Staining of the cells was visualized using a fluorescence microscope. The bar indicates 10 μm. (C) The ROS levels (DCFDA-F) in cells treated as in (B) were quantitatively measured using a cell imaging software program. (D-F) USP10-knockdown (sh-USP10-1) Jurkat cells and control (sh-NT) (D) or Jurkat cells (E,F) were infected with the indicated Tax lentiviruses. The infected cells were assessed for ROS production (DCFDA-F) using 5 μM of CM-H2DCFDA. Aliquots of the above-treated cells were subjected to a western blot analysis using anti-Tax, anti-USP10, and anti-α-tubulin antibodies. In all experiments, the values denote the mean ± SD; *P < .05; **P < .01; ***P < .001; NS indicates not statistically significant.
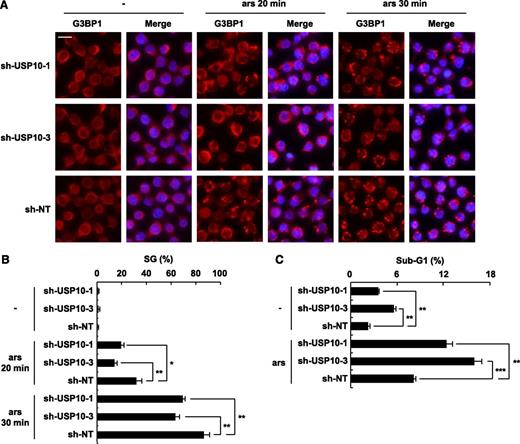
USP10 in T cells controls the SG-forming activity and sensitivity to arsenite-induced apoptosis. (A) USP10-knockdown Jurkat cells and control cells were treated with 0.25 mM of sodium arsenite for 0, 20, and 30 minutes and then were stained with anti-G3BP1 antibodies (red) and Hoechst33258 (blue). The bars indicate 10 μm. (B) The SG (%) is presented. (C) USP10-knockdown Jurkat cells and control cells were treated with 5 μM of sodium arsenite for 48 hours and then were stained with propidium iodide (PI). The proportion of the sub-G1 fraction was measured using flow cytometry. In all experiments, the values denote the mean ± SD; *P < .05; **P < .01; ***P < .001.
USP10 in T cells controls the SG-forming activity and sensitivity to arsenite-induced apoptosis. (A) USP10-knockdown Jurkat cells and control cells were treated with 0.25 mM of sodium arsenite for 0, 20, and 30 minutes and then were stained with anti-G3BP1 antibodies (red) and Hoechst33258 (blue). The bars indicate 10 μm. (B) The SG (%) is presented. (C) USP10-knockdown Jurkat cells and control cells were treated with 5 μM of sodium arsenite for 48 hours and then were stained with propidium iodide (PI). The proportion of the sub-G1 fraction was measured using flow cytometry. In all experiments, the values denote the mean ± SD; *P < .05; **P < .01; ***P < .001.
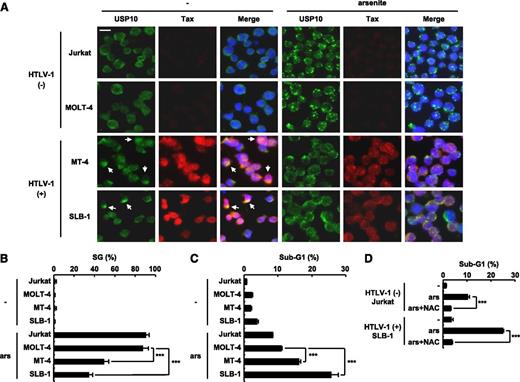
The level of arsenite sensitivity of HTLV-1–infected T cells correlates with the level of SG-forming activity. (A,B) HTLV-1–uninfected (Jurkat and MOLT-4) and HTLV-1–infected (SLB-1 and MT-4) T cells were treated with 0.5 mM of sodium arsenite for 30 minutes and then were stained with anti-USP10 antibodies (green), anti-Tax antibodies (red), and Hoechst33258 (blue). The arrows indicate cells with colocalization of USP10 and Tax. The bar indicates 10 μm in (A). The SG (%) in the indicated cells is presented in (B). (C) HTLV-1–uninfected (Jurkat and MOLT-4) and HTLV-1–infected (MT-4 and SLB-1) T cells were treated with 5 μM of sodium arsenite for 48 hours and then were stained with PI. The proportion of the sub-G1 population (apoptotic cells) was measured using flow cytometry. (D) Jurkat and SLB-1 cells were incubated with or without 10 mM of NAC, further treated with 5 μM of sodium arsenite for 48 hours, and stained with PI. The proportion of sub-G1 populations (%) was measured using flow cytometry. In all experiments, the values denote mean ± SD; ***P < .001.
The level of arsenite sensitivity of HTLV-1–infected T cells correlates with the level of SG-forming activity. (A,B) HTLV-1–uninfected (Jurkat and MOLT-4) and HTLV-1–infected (SLB-1 and MT-4) T cells were treated with 0.5 mM of sodium arsenite for 30 minutes and then were stained with anti-USP10 antibodies (green), anti-Tax antibodies (red), and Hoechst33258 (blue). The arrows indicate cells with colocalization of USP10 and Tax. The bar indicates 10 μm in (A). The SG (%) in the indicated cells is presented in (B). (C) HTLV-1–uninfected (Jurkat and MOLT-4) and HTLV-1–infected (MT-4 and SLB-1) T cells were treated with 5 μM of sodium arsenite for 48 hours and then were stained with PI. The proportion of the sub-G1 population (apoptotic cells) was measured using flow cytometry. (D) Jurkat and SLB-1 cells were incubated with or without 10 mM of NAC, further treated with 5 μM of sodium arsenite for 48 hours, and stained with PI. The proportion of sub-G1 populations (%) was measured using flow cytometry. In all experiments, the values denote mean ± SD; ***P < .001.
To delineate the domain of Tax that is responsible for the interaction with USP10, we examined the interactions between Tax mutants and USP10. Three of the mutants, TaxSH-1, TaxSH-2, and Tax62-353, exhibited minimal interaction with USP10 (Figure 2A-B). TaxSH-1 and TaxSH-2 have point mutations at Pro-58 to Ser and Leu-205 to Arg, respectively. Tax62-353 has an N-terminal deletion from amino acids 1 to 61, containing the zinc finger region. Therefore, both the N-terminal region and the central region of Tax are required for interaction with USP10. On the other hand, TaxM22 and Tax703 demonstrated equivalent USP10-binding activity to wild-type (WT) Tax and have point mutations at Gly-137 and Leu-138 and at Leu-319 and Leu-320, respectively (Figure 2A-B).
To identify the domain of USP10 responsible for the interaction with Tax, plasmids encoding various USP10 mutants were constructed (Figure 2C). An immunoprecipitation analysis of 293T cells showed that the deletion of amino acids 727 to 798 of the C-terminal region of USP10 prominently reduced binding with Tax, whereas the deletion of amino acids 1 to 116 of the N-terminal region, which are essential for the formation of SGs as well as binding with G3BP1 and PABP1,3 had a minimal effect on binding with Tax (Figure 2D), indicating that Tax interacts with amino acids 727 to 798 of USP10.
Binding of Tax with USP10 inhibits arsenite-induced SG formation
USP10 is a component of SGs that is involved in innate immunity against viral infections.8 Intriguingly, Tax inhibits SG formation.14 Therefore, we next investigated whether USP10 is involved in the Tax-mediated inhibition of SG formation. Arsenite, a prooxidant well known to stimulate SG formation, induced SGs in 293T cells, and USP10 was predominantly detected in the formed SGs (Figure 3A). The 293T cells were then transiently transfected with expression vectors encoding WT Tax, Tax62-353, TaxSH-1, or TaxSH-2, treated with arsenite and stained with anti-USP10 antibodies. WT Tax inhibited arsenite-induced SG formation (as represented by the arrow in Figure 3B). This is consistent with the findings of a previous study.14 In addition, 2 Tax mutants (TaxM22 and Tax703) that are capable of interacting with USP10 also reduced arsenite-induced SG formation (Figure 3D). Of the 3 Tax mutants defective in USP10 binding, Tax62-353 was completely unable to interfere with SG formation. In contrast, the other 2 Tax mutants (TaxSH-1 and TaxSH-2) inhibited SG formation; however, the inhibitory activity was half that of WT Tax (Figure 3B-C). These results suggest that Tax inhibits SG formation and that this inhibition is partly mediated through the interaction between Tax and USP10.
Tax augments arsenite-induced apoptosis of 293T cells
Because SGs inhibit arsenite-induced apoptosis,3,22,23 we next examined whether Tax affects the sensitivity of 293T cells to arsenite-induced apoptosis. 293T cells were transfected with expression vectors encoding Tax and its mutants, and the cells were treated with 0.5 mM of arsenite for 80 minutes, washed with PBS, and further cultured for 1 hour. The cells were then stained with anti-Tax antibodies and Hoechst33258 (supplemental Figure 1). The apoptotic cells were monitored by detecting condensed nuclei in the Tax-expressing cells (Figure 3E). Tax augmented the arsenite-induced apoptosis of 293T cells, and this apoptosis was inhibited by an antioxidant (NAC) (Figure 3F). On the other hand, the augmentation of apoptosis induced by TaxSH-1 and TaxSH-2 in the 293T cells was approximately half that induced by WT Tax, and Tax62-353 had a minimal effect on apoptosis (Figure 3E). These results suggest that Tax, at least in part through its interaction with USP10, augments arsenite-induced apoptosis of 293T cells, and that this apoptosis augmentation correlates with the SG inhibitory activity.
Tax stimulates ROS production via USP10 in T cells
We recently showed that USP10 downregulates steady-state ROS production in several epithelial cell lines and inhibits arsenite-induced ROS production.3 To elucidate the role of USP10 in ROS production in T cells, we knocked down the USP10 expression in a human T-cell line (Jurkat) using short hairpin RNA (shRNA) (Figure 4A) and measured the ROS levels using staining with CM-H2DCFDA, a redox-sensitive probe (Figure 4B). Two USP10-knockdown cells targeting different regions of USP10 messenger RNA produced more ROS than the control cells (Figure 4C). These results suggest that endogenous USP10 in T cells downregulates ROS production under steady-state conditions.
Tax stimulates ROS production in T cells.24 To examine whether USP10 is involved in Tax-induced ROS production in T cells, USP10-knockdown (sh-USP10-1) Jurkat and control cells (sh-NT) were infected with Tax-encoding lentivirus, and the cells were assessed for ROS production. Tax elevated ROS production in the control cells; however, this activity was abrogated by the knockdown of USP10 (Figure 4D). Two Tax mutants, TaxSH-1 and TaxSH-2, defective for USP10 interactions were unable to elevate ROS production (Figure 4D). In addition, 2 other Tax mutants (TaxM22 and Tax703) that are active for USP10 binding failed to elevate ROS production (Figure 4E). Whereas WT Tax activates the transcription of cellular genes through 2 transcription factors, NF-κB and CREB, TaxM22 is inactive for NF-κB–dependent transcriptional activation, and Tax703 exhibits a weak degree of CREB-dependent activation.25,26 Therefore, these results suggest that transcriptional activation of cellular genes by Tax is required for the production of ROS induced by Tax. In addition, TaxΔC without PBM did not stimulate ROS production (Figure 4F). Therefore, PBM and its binding proteins are also required for Tax to stimulate ROS production. It should be noted that TaxSH-1 defective for the USP10 interaction possesses PBM and activates CREB- and NF-κB–dependent transcription equivalent to WT Tax. Taken together, these results indicate that Tax stimulates ROS production through combined actions by interacting with USP10 and modulating the activities of the NF-κB, CREB, and PBM pathways.
USP10 knockdown in T cells reduces SG-forming activities and augments sensitivity to arsenite-induced apoptosis
We next investigated the roles of USP10 in arsenite-induced SG formation and apoptosis in T cells. High-dose arsenite (0.25 mM) induced SG formation in 2 distinct USP10-knockdown Jurkat cells; however, the level of SGs was less than that observed in the control cells (Figure 5A-B). In addition, knockdown of USP10 augmented the apoptosis of Jurkat cells treated with or without a low dose of arsenite (5 μM) (Figure 5C). It should be noted that human T-cell lines are more sensitive to arsenite-induced apoptosis than adherent cell lines, including 293T cells, and even a concentration of arsenite (5 μM) that does not induce the formation of visible SGs can still induce apoptosis in T-cell lines, but not adherent cell lines. These results indicate that USP10 in T cells augments SG formation and reduces arsenite-induced apoptosis.
Augmented arsenite-induced apoptosis of HTLV-1–infected T cells is associated with a reduced level of SG-forming activity
A recent study showed that combination therapy containing arsenic trioxide, interferon-α, and zidovudine exhibits promising therapeutic effects on some forms of ATL.16 In addition, arsenite induces apoptosis in HTLV-1–infected T-cell lines more so than in HTLV-1–uninfected T-cell lines.27 Therefore, we next examined whether the high sensitivity of HTLV-1–infected T-cell lines to arsenite is related to the level of SG-forming activity. HTLV-1–infected T-cell lines treated with a low dose of arsenite exhibited more apoptosis than the HTLV-1–uninfected cell lines (Figure 6C). This apoptosis was inhibited by NAC (Figure 6D). In addition, high-dose arsenite induced SG formation in HTLV-1–infected T-cell lines, and the amount of SGs was lower than that observed in the HTLV-1–uninfected cell lines (Figure 6A-B). Moreover, USP10 knockdown in the HTLV-1–infected T-cell line MT-4 induced less arsenite-induced SG formation, more ROS production, and more arsenite-induced apoptosis than the control USP10-competent MT-4 cells (supplemental Figure 2). Collectively, these results indicate that USP10 inhibits ROS production and apoptosis in HTLV-1–infected T cells and that the inhibition correlates with the SG-forming activity.
Arsenic trioxide, an anhydrous form of arsenite, has been used as a drug against ATL in place of sodium arsenite.28 To confirm that arsenic trioxide also exhibits similar activities to arsenite, we treated HTLV-1–uninfected (Jurkat) and infected (SLB-1) T-cell lines with arsenic trioxide. Arsenic trioxide (0.1-1.0 mM) induced SG formation in both the Jurkat and SLB-1 cells; however, the level of SG-forming activity was lower in the SLB-1 cells than in the Jurkat cells (supplemental Figure 3A). Moreover, the arsenic trioxide-induced apoptosis of the SLB-1 cells was significantly greater than that of the Jurkat cells, and the apoptosis was abrogated by treatment with NAC (supplemental Figure 3B-C). These results suggest that arsenic trioxide induces the ROS-dependent apoptosis of HTLV-1–infected T cells via the same mechanism as sodium arsenite (Figure 6).
Approximately half of leukemic cells in patients with ATL do not express a functional tax gene.29 Therefore, we next examined whether 3 ATL cell lines established from patients with ATL are sensitive to arsenite-induced apoptosis. Three ATL cell lines with or without a minimal amount of a Tax protein expression demonstrated different sensitivities to arsenite-induced apoptosis, and the level of sensitivity correlated inversely with the level of SG-forming activity (Figure 7A-B). These results suggest that sensitivity to arsenite-induced apoptosis in ATL cells is also regulated by SG-forming activities. To extend this hypothesis into cell types other than T cells, we characterized the sensitivity to arsenite-induced apoptosis and the SG-forming activity of leukemia/lymphoma cell lines unrelated to ATL. The 3 B-cell lines (Ramos, Daudi, and BJAB) were derived from Burkitt lymphoma (Figure 7C-D); the next one (NB4), from promyeloid leukemia; and the last one (THP-1), from acute monocytic leukemia (Figure 7E-F). They exhibited distinct sensitivities to arsenite-induced apoptosis, and the level of sensitivity correlated inversely with the level of SG-forming activity. These results further support the idea that arsenite-induced SG formation of various hematopoietic leukemic cells correlates inversely with sensitivity to arsenite-induced apoptosis.
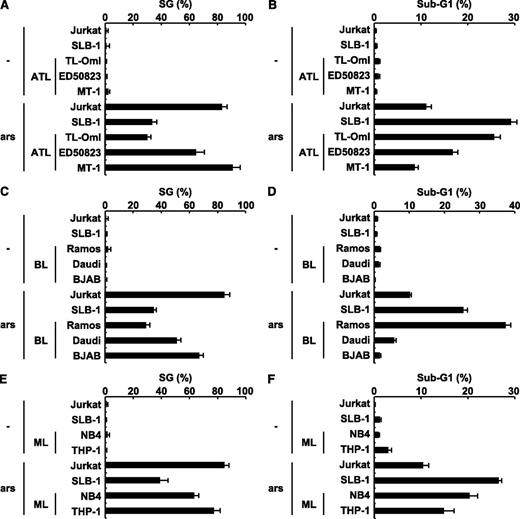
The level of sensitivity to arsenite-induced apoptosis in ATL, Burkitt lymphoma, and myeloid leukemia cell lines correlates with the level of SG-forming activity. (A) HTLV-1–uninfected (Jurkat), HTLV-1–infected (SLB-1; used as a positive control), and ATL-derived (TL-OmI, ED50823, and MT-1) T-cell lines were treated with 0.5 mM of sodium arsenite for 30 minutes and then were stained with anti-USP10 antibodies and Hoechst33258. The SG (%) is presented. (B) HTLV-1–uninfected (Jurkat), HTLV-1–infected (SLB-1), and ATL-derived (TL-OmI, ED50823, and MT-1) T-cell lines were treated with 5 μM of sodium arsenite for 48 hours and then were stained with PI. The proportion of the sub-G1 population (%) (apoptotic cells) was measured using flow cytometry. (C-F) The levels of arsenite-induced SG formation (%) and the proportions of the sub-G1 population (%) in the Burkitt lymphoma (C,D) and myeloid leukemia (ML) cell lines (E,F) were assessed.
The level of sensitivity to arsenite-induced apoptosis in ATL, Burkitt lymphoma, and myeloid leukemia cell lines correlates with the level of SG-forming activity. (A) HTLV-1–uninfected (Jurkat), HTLV-1–infected (SLB-1; used as a positive control), and ATL-derived (TL-OmI, ED50823, and MT-1) T-cell lines were treated with 0.5 mM of sodium arsenite for 30 minutes and then were stained with anti-USP10 antibodies and Hoechst33258. The SG (%) is presented. (B) HTLV-1–uninfected (Jurkat), HTLV-1–infected (SLB-1), and ATL-derived (TL-OmI, ED50823, and MT-1) T-cell lines were treated with 5 μM of sodium arsenite for 48 hours and then were stained with PI. The proportion of the sub-G1 population (%) (apoptotic cells) was measured using flow cytometry. (C-F) The levels of arsenite-induced SG formation (%) and the proportions of the sub-G1 population (%) in the Burkitt lymphoma (C,D) and myeloid leukemia (ML) cell lines (E,F) were assessed.
Discussion
In our study, we identified USP10 as a novel binding protein of HTLV-1 Tax in T cells and found that Tax inhibits at least 3 USP10-associated functions, namely, SG formation, ROS suppression, and inhibition of apoptosis. Under various stress conditions, including viral infection, USP10 can prevent the emergence of cells with stress-induced ROS-dependent DNA and protein alterations.3 Therefore, our present findings suggest that Tax, by interacting with USP10, augments ROS-dependent alterations, such as DNA damage, in HTLV-1–infected T cells, thereby promoting leukemogenesis.
Two Tax mutants (TaxSH-1 and TaxSH-2) defective for USP10 binding inhibited SG formation; however, the inhibition achieved by these mutants was half that of WT Tax. Legros et al demonstrated that Tax inhibits SG formation by binding to histone deacetylase 6 (HDAC6), a component of SGs.14 Therefore, both USP10 and HDAC6 are likely to play a role in the Tax-induced inhibition of SG formation. On the other hand, HDAC6 is not likely to mediate Tax-induced ROS production because, unlike WT Tax, TaxSH-1 and TaxSH-2 have a minimal effect on ROS production.
The activities of the Tax mutants suggest that multiple functions of Tax, in addition to the inhibition of USP10 functions, are involved in the stimulation of ROS production. Two Tax mutants (TaxM22 and Tax703), which are defective for NF-κB- and CREB-dependent transcriptional activation, respectively, both failed to elevate ROS production (Figure 4), suggesting that Tax stimulates ROS generation via gene(s) regulated by NF-κB and CREB. Indeed, NF-κB can activate prooxidant genes, such as the reduced NAD phosphate oxidase NOX2.30,31 In addition, PBM was required for Tax to stimulate ROS production (Figure 4). Tax, acting through PBM, interacts with several PDZ domains containing proteins, including Dlg1, Scribble, and MAGI-1, then inactivates the functions of these PDZ proteins by altering subcellular localization.32-35 The PDZ proteins localize near the plasma membrane and form a complex with several membrane receptors, then positively or negatively control signals from corresponding membrane receptors.36,37 Collectively, we tentatively propose the following hypothesis of how Tax stimulates ROS production in T cells. Although Tax, acting through the NF-κB, CREB, and PBM pathways, induces the expression of prooxidant gene(s), by acting through USP10, it blocks the antioxidant response, thereby prolonging the production of ROS. A further analysis is, however, required to understand the precise mechanisms underlying how Tax stimulates ROS production in T cells.
Tax is an ubiquitinated protein, and the ubiquitination controls the stability and transcriptional activity of Tax.38,39 Although USP10 is a Tax-interacting deubiquitinase, the overexpression of USP10 in 293T cells minimally affects the ubiquitination of Tax (H.M, M.T., and M.F., unpublished observations). In this aspect, several proteins have been shown to modulate the ubiquitination of Tax. For instance, USP20 directly deubiquitinates Tax and suppresses the NF-κB activation induced by Tax.40 In addition, the metalloprotease STAM-binding protein-like 1 indirectly induces the deubiquitination of Tax, thereby stabilizing Tax and promoting its nuclear export.41 On the other hand, Tax is ubiquitinated by The Really Interesting New Gene Finger Protein 4, and this ubiquitination is required for cytoplasmic localization and the activation of NF-κB.42 Therefore, further analyses of these cellular proteins, including USP10, will clarify the precise mechanisms underlying the functions of Tax in the pathogenesis of HTLV-1.
A previous study showed that USP10 inhibits arsenite-induced ROS-dependent apoptosis in MEFs and that this inhibitory activity is mediated by the formation of USP10-containing SGs.3 In our study, unlike MEFs, USP10 inhibited arsenite-induced ROS-dependent apoptosis in a human T-cell line, even at a concentration of arsenite that does not provoke visible SG formation. These results suggest 2 possible mechanisms. USP10 may block ROS-dependent apoptosis without forming SGs in T cells. Alternatively, a low concentration of arsenite may induce small SGs undetectable by the present assay method, and such undetectable small SGs may inhibit apoptosis. We are currently studying how USP10 inhibits arsenite-induced apoptosis without visible SGs in T cells.
A clinical trial has indicated that combination therapy containing arsenic trioxide, interferon α, and zidovudine exhibits promising antileukemia activity against some forms of ATL16 ; however, it is unclear which patients are sensitive to this chemotherapy. Our present study suggests that Tax augments the sensitivity to arsenite-induced apoptosis of cells. Takeda et al showed that 66% of ATL cells exhibit inactivation of the tax gene because of mutation or DNA methylation.29 Therefore, the expression of the tax gene in ATL cells is likely to be a factor controlling the sensitivity of ATL cells to chemotherapy containing arsenic. In addition, a single ATL-derived cell line without a Tax expression (TL-OmI) demonstrated higher sensitivity to arsenite-induced apoptosis than the other 2 ATL cell lines (Figure 7), and the different levels of arsenite sensitivity correlated with the level of SG-forming activity in the cells. Therefore, factor(s) other than Tax control(s) the sensitivity of ATL cells to arsenite-induced apoptosis, and this sensitivity might also be related to the SG-forming activity. Because the SG-forming activity is regulated by various host factors, such as G3BP1,6 G3BP2,43 and HDAC6,22 these host proteins may be involved in the high arsenite-induced apoptosis of ATL cell lines without a Tax expression.
Arsenic trioxide is a highly effective chemotherapy for promyelocytic leukemia (PML).28 Arsenic trioxide induces ROS-dependent apoptosis in several PML cell lines, including NB4, in vitro.44,45 In our study, in addition to ATL cell lines, 5 leukemia cell lines derived from PML, myeloid leukemia, and Burkitt lymphoma exhibited distinct sensitivities to arsenite-induced apoptosis, and the level of sensitivity correlated with the degree of arsenite-induced SG formation. Therefore, understanding how USP10 and SGs control sensitivity to arsenite-induced apoptosis in hematopoietic cells will provide useful information for developing more effective chemotherapies for the treatment of hematopoietic malignancies, including ATL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr H. Miyoshi (RIKEN Tsukuba Institute, Japan) for providing the lentiviral packaging plasmids and Dr M. Masuko (Niigata University, Japan) for providing NB4 cells. The authors also thank the Takeda Pharmaceutical Company for providing recombinant human IL-2 and M. Tobimatsu for technical assistance.
This work was supported in part by a Grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Grant for the Promotion of Niigata University Research Project.
Authorship
Contribution: M.T. performed most of the experiments and contributed to the data interpretation; M.H. performed the mass spectrometry analysis and contributed to the data interpretation; G.N.M., H.M., and M.Y. assisted in conducting the experiments; Y.T. provided the anti-Tax antibody; and M.F. contributed to the conception, design, and data interpretation of the study and wrote and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masahiro Fujii, Division of Virology, Niigata University Graduate School of Medical and Dental Sciences, Niigata 951-8510, Japan; e-mail: fujiimas@med.niigata-u.ac.jp.